Rhodamine B derivative, preparation method and application of rhodamine B derivative serving as fluorescent probe
A fluorescent probe and derivative technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of arterial elasticity reduction, cholesterol increase, blood pressure increase, etc., achieving strong selectivity, high sensitivity, The effect of easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
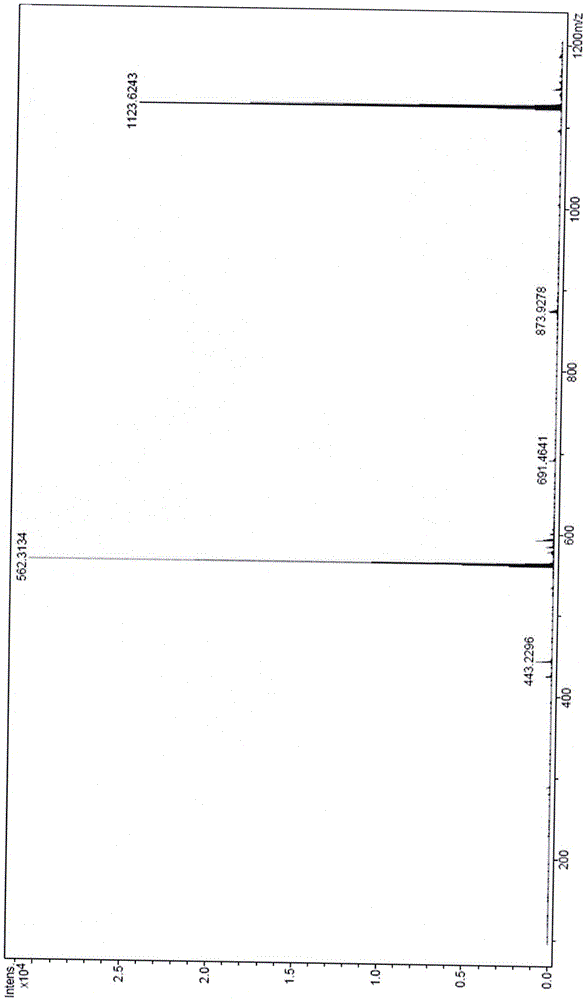
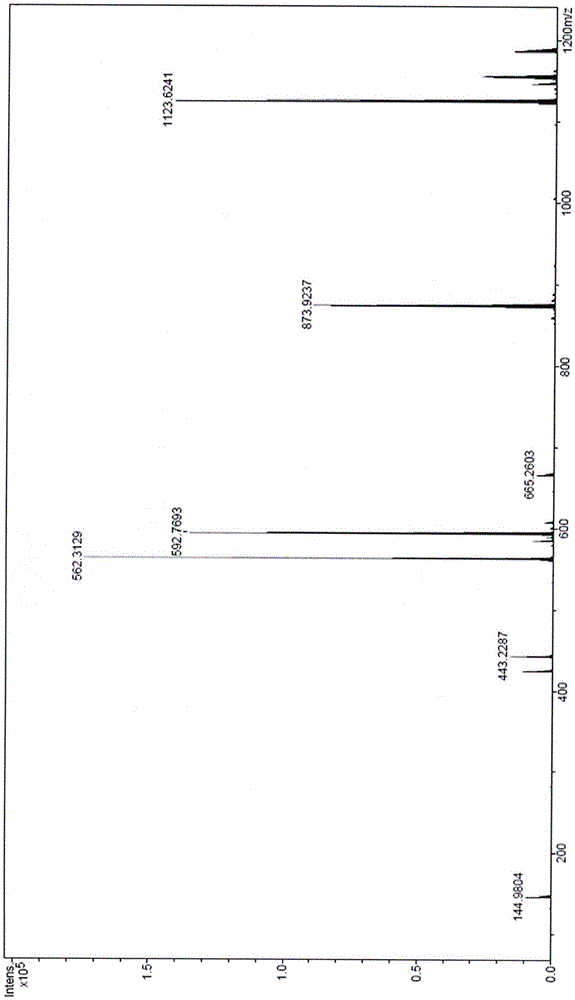
Image
Examples
Embodiment 1
[0028] Synthesis of Rhodamine B Derivatives
[0029]
[0030] Dissolve 0.167g of 3,5-dimethyl-pyrrole-2-carbaldehyde in 10mL of absolute ethanol, then add 0.46g of Rhodamine B hydrazide, and stir under reflux for 24 hours under normal pressure. After cooling to room temperature, a large amount of solids are precipitated. After filtering, the filter residue was washed with absolute ethanol to obtain a white solid, which was the target product, and the yield of the target product was 76%.
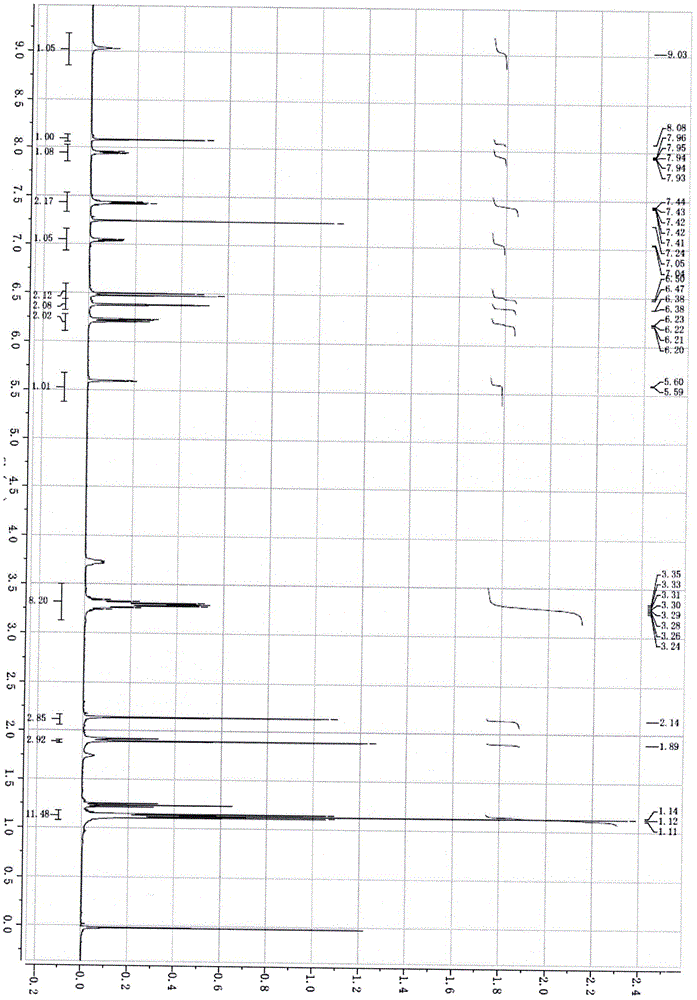
[0031] Adopt nuclear magnetic resonance instrument to carry out nuclear magnetic resonance analysis to the rhodamine B derivative that makes, calculation result is as follows:
[0032] 1HNMR (400MHz, CDCl 3 ), δ (ppm): 9.03 (s, 1H, NH), 8.08 (s, 1H, CH=N), 7.93-7.96 (m, 1H, Aryl-H), 7.41-7.42 (m, 2H, Aryl- H), 7.04-7.05 (m, 1H, Aryl-H), 6.47-6.50 (d, 2H, Aryl-H), 6.38 (s, 2H, Aryl-H), 6.21-6.23 (m, 2H, Aryl-H), H), 5.09-5.60 (d, 1H, pyrrole-CH), 3.24-3.35 (q, 8H, 4CH 2 ), 2.14 (s, 3H, ...
Embodiment 2
[0035] Dissolve 12.32g of 3,5-dimethyl-pyrrole-2-carbaldehyde in 0.5L of absolute ethanol, then add 45.5g of rhodamine B hydrazide, and stir under reflux for 12 hours under normal pressure. After cooling to room temperature, a large amount of solids are precipitated. After pressure filtration, the filter residue was washed with 75% ethanol solution to obtain a white solid, which was the target product, and the yield of the target product was 72.5%.
Embodiment 3
[0037] Dissolve 9.823g of 3,5-dimethyl-pyrrole-2-carbaldehyde in 0.4L of absolute ethanol, then add 36.21g of rhodamine B hydrazide, and stir under reflux for 12 hours under normal pressure. After cooling to room temperature, a large amount of solids are precipitated. After pressure filtration, the filter residue was washed with 75% ethanol solution to obtain a white solid, which was the target product, and the yield of the target product was 75.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com