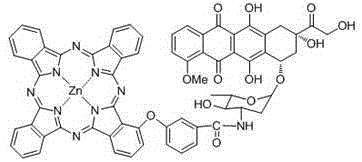
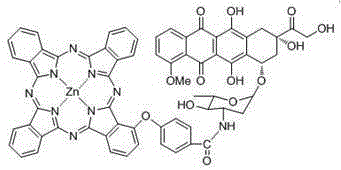
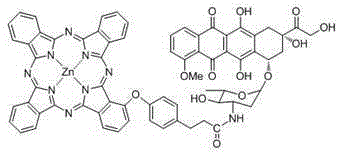
Zinc(II) carboxyl phthalocyanine and adriamycin conjugate and preparation and application thereof
A technology of zinc phthalocyanine and doxorubicin, which is applied in the field of carboxyzinc phthalocyanine-doxorubicin conjugates and their preparation and application, which can solve the problems of lack of efficient combination drugs, clinical application limitations, and high skin phototoxicity. problem, to achieve the effect of good clinical application prospect, good stability and high photosensitization ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of carboxyl phthalocyanine zinc-doxorubicin conjugate comprises the following steps:
[0030] With zinc phthalocyanine and doxorubicin hydrochloride as reactants, N, N-dimethylformamide as solvent, in 1-ethyl-(3-dimethylaminopropyl) carbodiimide In the presence of hydrochloride, 1-hydroxybenzotriazole, 4-dimethylaminopyridine and under the protection of nitrogen, continue to stir and react at room temperature -35°C for 8-24h, and then purify by extraction method or solvent method, and column chromatography Carboxyzinc phthalocyanine-doxorubicin conjugate;
[0031] In the above reaction, the molar ratio of carboxyl zinc phthalocyanine and doxorubicin hydrochloride is 1:1-2; 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride, The amount of 1-hydroxybenzotriazole and 4-dimethylaminopyridine is 1-3 mmol per mmol of carboxyzinc phthalocyanine; the amount of solvent is 5-10 mL.
[0032] The carboxyl phthalocyanine zinc of the present invention...
Embodiment 1
[0048] Compound (1), namely 1-(3-carboxyphenoxy)zinc phthalocyanine represented by formula (1):
[0049] ;
[0050] (1)
[0051] The preparation method comprises the following steps:
[0052] (1) Prepare 3-(3-carboxyphenoxy)phthalonitrile with the following structure: ;
[0053] Take 3-hydroxybenzoic acid (15mmol) and 3-nitrophthalonitrile (15mmol) as reactants, anhydrous DMSO as solvent (30ml), in the presence of potassium carbonate (45mmol) and nitrogen protection, stir at room temperature The reaction was carried out for 30 hours, and the end point of the reaction was monitored by thin layer chromatography. The reaction mixture was suction-filtered with a sand core funnel, the filtrate was collected, and the filtrate was added to 500ml of ice-water mixture, adjusted with 1M hydrochloric acid solution until the solution was acidic, and a large amount of precipitate was precipitated, left to stand, and washed repeatedly with a microporous organic filter membrane. The ...
Embodiment 2
[0059] Compound (2), namely 1-(4-carboxyphenoxy)zinc phthalocyanine represented by formula (2):
[0060] ;
[0061] (2)
[0062] The preparation method comprises the following steps:
[0063] (1) Prepare 3-(4-carboxyphenoxy)phthalonitrile with the following structure: ;
[0064] With p-hydroxybenzoic acid (15mmol) and 3-nitrophthalonitrile (15mmol) as reactants, with anhydrous DMSO as solvent (30ml), in the presence of potassium carbonate (45mmol) and under the protection of nitrogen, the reaction was stirred at room temperature At 30 hours, the reaction endpoint was monitored by thin layer chromatography. The reaction mixture was suction-filtered with a sand core funnel, the filtrate was collected, and the filtrate was added to 500ml of ice-water mixture, adjusted with 1M hydrochloric acid solution until the solution was acidic, and a large amount of precipitate was precipitated, left to stand, and washed repeatedly with a microporous organic filter membrane. The next...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com