Method for regenerating ATP using rationally designed enzyme
A rational polyphosphate kinase technology, applied in biochemical equipment and methods, enzymes, transferases, etc., can solve problems such as inability to couple enzymes at room temperature, high degree of polyphosphate polymerization, and difficulty in obtaining polyphosphate. To achieve the effect of cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Mutation and cloning of rationally designed polyphosphate kinase gene from Sinorhizobium meliloti
[0027] The polyphosphokinase gene ppk from Sinorhizobium meliloti was mutated into ppk(ket) by site-directed mutagenesis. Using the strain's original gene recombination vector as a template, design primers according to the GenBank sequence, and operate according to the instructions of the TaKaRaMutanBESTkit kit.
[0028] The primers used for site-directed mutagenesis are as follows:
[0029] F1:GCGATCAAAGCGACGACGGAAAATATGAACCCCCGCTC
[0030] R1: ACCACCCTTGCCGGCAGCGT
[0031] F2: GCGCGCACCGTCGCACTGACGAAACCGA
[0032] R2: GGAGCGGGGGTTCATATT
[0033] The size of the mutated recombinant vector fragment is about 6000bp, see the nucleic acid electrophoresis figure 1 .
Embodiment 2
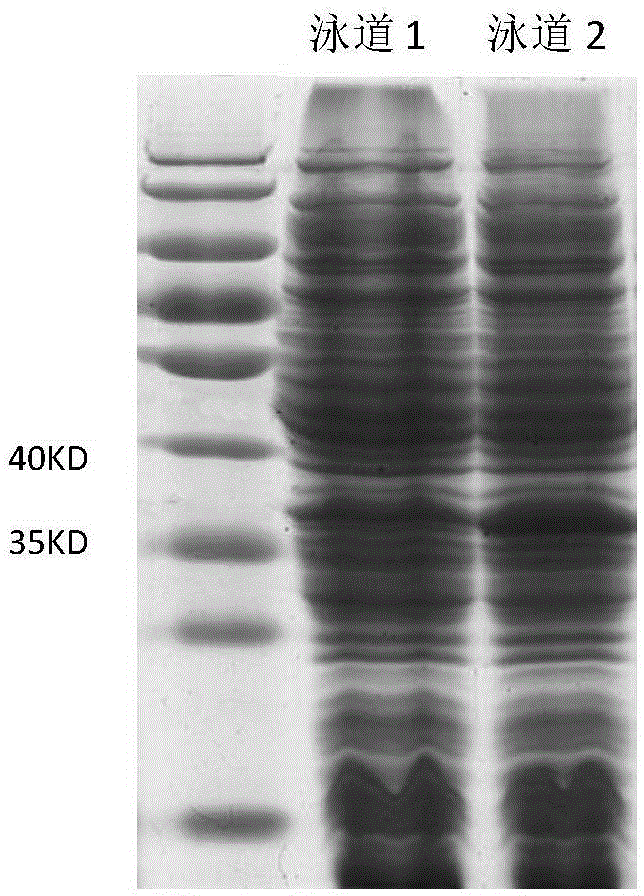
[0035] Transform the constructed expression vector, pET22b-ppk (ket) plasmid, into BL21 (DE3) Escherichia coli, use LB medium after picking the bacteria, and grow at 37°C until the OD600 is about 0.4-1.0, then add 0.1mM-1mM Induced by IPTG, intracellular expression was performed at 30°C. The vector pET22b was transformed into BL21(DE3) Escherichia coli, and the expression was induced and expressed under the same conditions as above, and the expressed crude enzyme solution was subjected to protein electrophoresis to verify the expression results. The protein electrophoresis was as follows: figure 2 As shown, the protein content measured by Coomassie brilliant blue method was 8.4g / L.
Embodiment 3
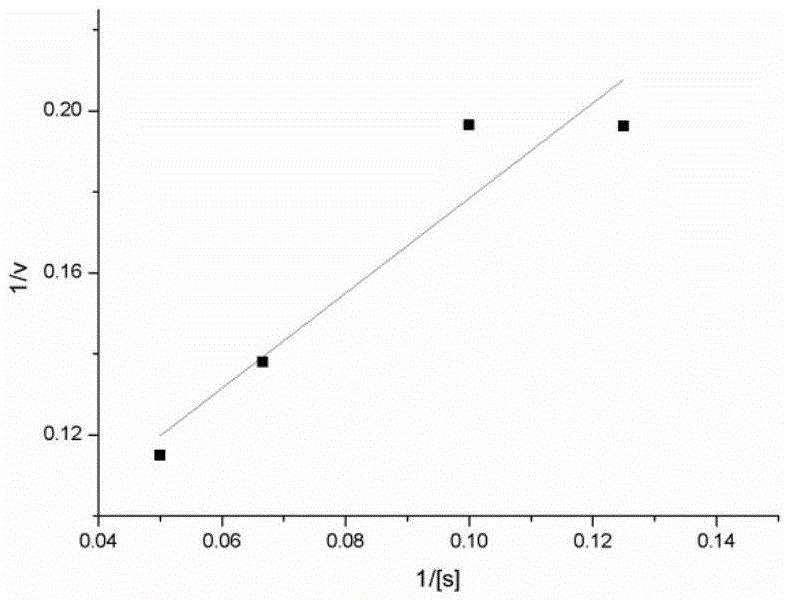
[0037] The kinetic parameters of PPK enzyme were measured when tetrapolyphosphate was used as substrate. Six groups of experimental systems 1 numbered 1, 2, 3, 4, 5, and 6 were set up, and the concentrations of tetrapolyphosphate in the system were 2mM, 4mM, 8mM, 10mM, 15mM, and 20mM, respectively. Add ADP corresponding to twice the concentration of hexametaphosphate, 30mM MgCl 2 , 200ul of PPK crude enzyme solution (containing 0.12mg of protein), adding different amounts of 3M pH8.0 Tris-HCl to adjust the pH of the system to 8.0, system 1 was reacted at 37 degrees for 5 minutes and then placed in a 60 degrees water bath for 20 minutes to inactivate the PPK enzyme. Take the corresponding supernatant and add it to NADPH reaction system 2, marked as 1, 2, 3, 4, 5, 6. The corresponding glucose concentration of each group in system 2 is 1mM, 2mM, 3mM, 4mM, 5mM, 7mM glucose and NADP, and the amount of hexokinase and glucose-6-phosphate dehydrogenase in the corresponding numbered e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com