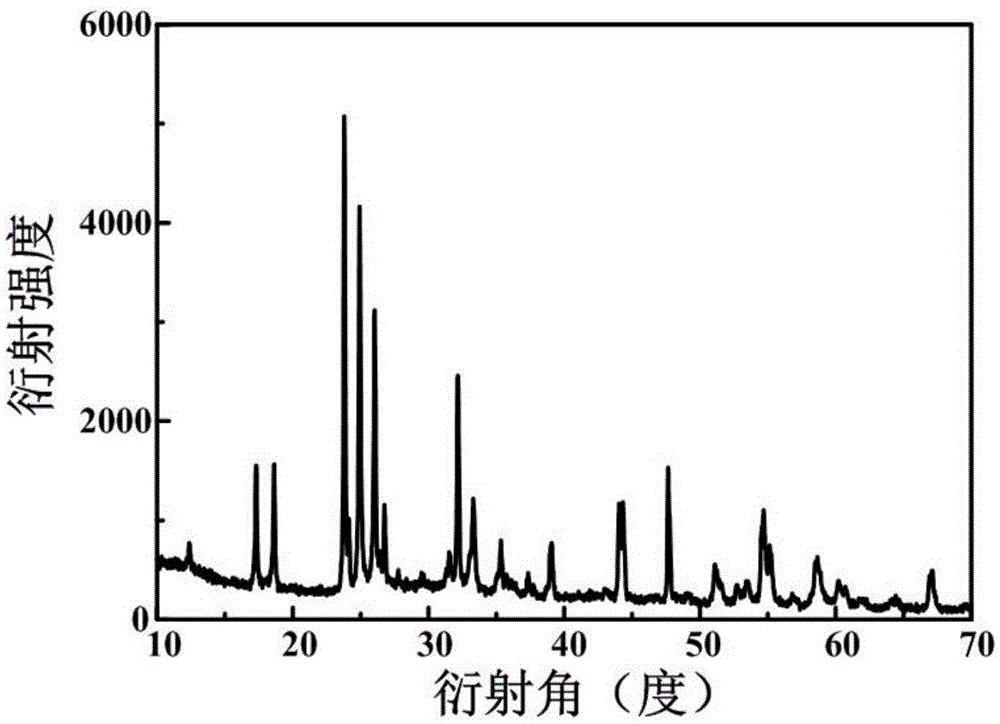
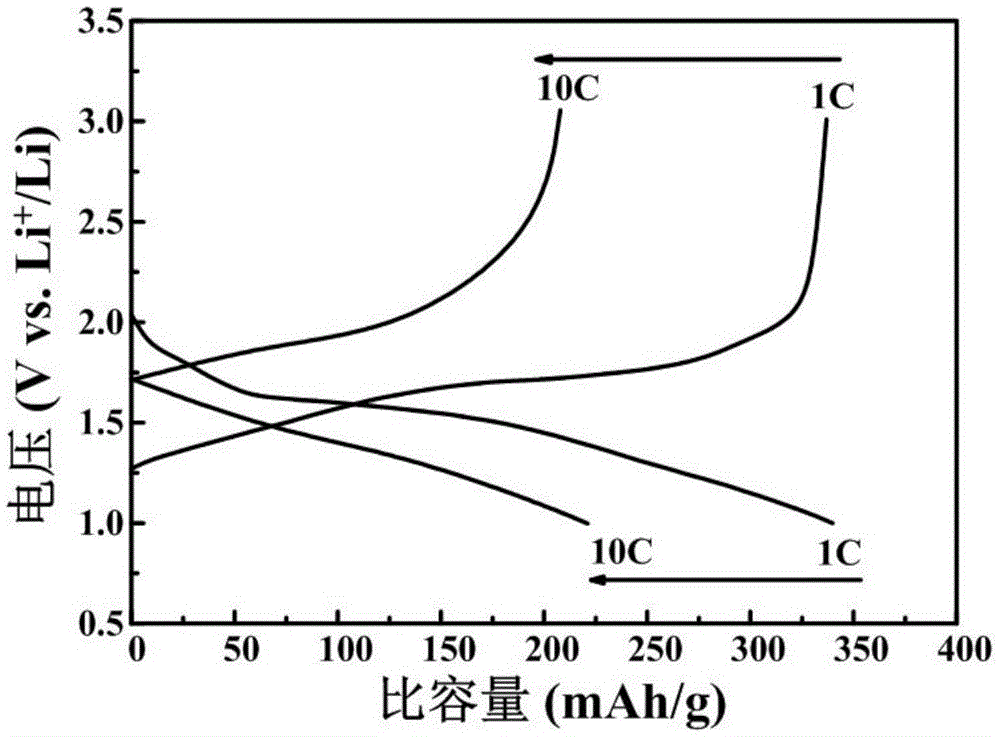
Preparation method of lithium ion battery negative material titanium niobate
A technology for lithium ion batteries and negative electrode materials, applied in the field of electrochemistry, can solve the problems affecting the electrochemical performance of titanium niobate materials, long diffusion distance, large particles of titanium niobate materials, etc., and achieves convenient industrial production, simple steps, and easy effect of operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Dissolve niobium ethoxide and butyl titanate in a certain amount of acetic acid according to the atomic ratio niobium / titanium=5.82:1, and then mix slowly, where the niobium ion concentration is 0.02mol / L;
[0033] 2) Transfer the mixture obtained in step 1) to the reaction kettle, place it in a constant temperature drying oven, and react at 160°C for 48 hours;
[0034] 3) Naturally cool to room temperature, wash the obtained precipitate with deionized water and ethanol three times, and then dry at 73°C to obtain a powder;
[0035] 4) The powder obtained in step 3) is fired at 1100°C for 12 hours to obtain titanium niobate (TiNb 6 O 17 )material.
Embodiment 2
[0037] 1) Dissolve niobium pentachloride and titanium sulfate in a certain amount of acetic acid according to the atomic ratio niobium / titanium=5.63:1, and then slowly mix them, where the niobium ion concentration is 1.1mol / L;
[0038] 2) Transfer the mixture obtained in step 1) to the reaction kettle, place it in a constant temperature drying oven, and react at 245°C for 36 hours;
[0039] 3) Naturally cool to room temperature, wash the obtained precipitate with deionized water and ethanol three times, and then dry at 80°C to obtain a powder;
[0040] 4) The powder obtained in step 3) is calcined at 830°C for 24 hours to obtain titanium niobate (TiNb 6 O 17 )material.
Embodiment 3
[0042] 1) Dissolve niobium ethoxide and titanium tetraisopropoxide in a certain amount of acetic acid according to the atomic ratio niobium / titanium=5.2:1, and then slowly mix, where the niobium ion concentration is 0.002mol / L;
[0043] 2) Transfer the mixture obtained in step 1) to the reaction kettle, place it in a constant temperature drying oven, and react at 60°C for 48 hours;
[0044] 3) Naturally cool to room temperature, wash the obtained precipitate with deionized water and ethanol three times, and then dry at 111°C to obtain a powder;
[0045] 4) The powder obtained in step 3) is fired at 500°C for 48 hours to obtain titanium niobate (TiNb 6 O 17 )material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com