Flunitrazepam sublingual tablet preparation and preparation method thereof
A technology of flunitrazepam and sublingual tablet, applied in the field of flunitrazepam sublingual tablet preparation and preparation thereof, can solve the problems of being difficult to achieve rapid onset of effect, irritating, troublesome intramuscular injection, etc. And easy to carry, easy to take, complete and rapid effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
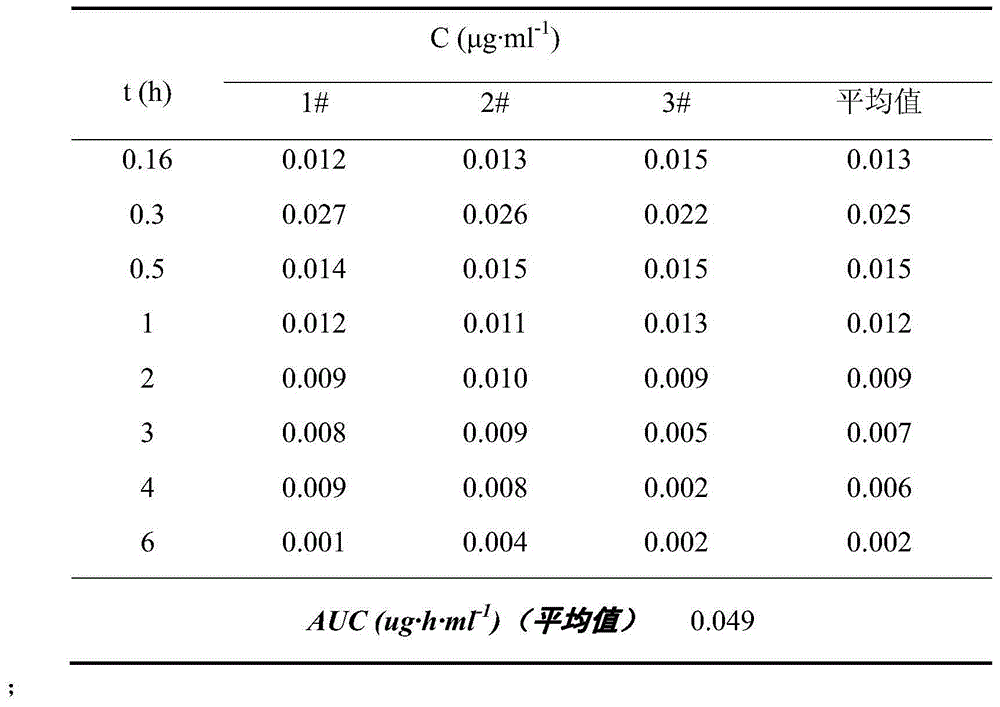
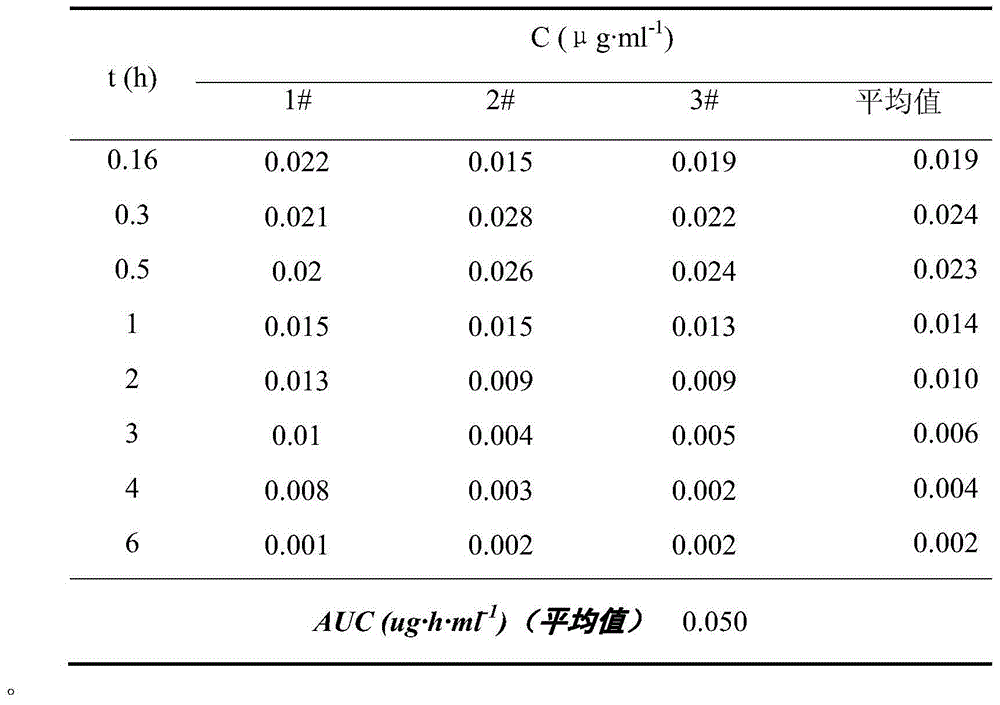
Image
Examples
preparation example Construction
[0041] In a more specific example, the preparation method of flunitrazepam sublingual tablet comprises the following steps:
[0042] Step 1: Mix sorbitol and polyethylene glycol 6000 evenly, and pass through a sieve of 80 mesh or 100 mesh;
[0043] Step 2: Dissolving polyvinylpyrrolidone K30 and caprylic capric acid polyethylene glycol glyceride in an appropriate amount of water to form a solution, the concentration of polyvinylpyrrolidone K30 in the aqueous solution can be 15-40 g / mL;
[0044] The third step: dissolving flunitrazepam in the second step solution, adding the above-mentioned powder to prepare soft material, crossing an appropriate mesh screen (such as 20 mesh sieve) to make granules, oven or fluidized bed drying (drying) The temperature is, for example, 40-60°C) and then sizing (for example, sizing through a 20-mesh sieve);
[0045] Step 4: Add magnesium stearate to the dry granules, mix well, and press into tablets.
[0046] Adopt technology of the present in...
Embodiment 1
[0053] Flunitrazepam
[0055] Mix sorbitol and polyethylene glycol 6000 evenly; dissolve polyvinylpyrrolidone K30 and caprylic capric acid macrogolglyceride in 20mL water to form solution A, then dissolve flunitrazepam in solution A, add the above powder Prepare the soft material in the middle, pass through a 20-mesh sieve to make granules, dry at 40-60°C, granulate with a 20-mesh sieve, add magnesium stearate to the dry granules, mix well, and press into tablets.
Embodiment 2
[0057] Flunitrazepam
[0058] Mix sorbitol and polyethylene glycol 6000 evenly; dissolve polyvinylpyrrolidone K30 and caprylic capric acid macrogolglyceride in 20mL water to form solution A, then dissolve flunitrazepam in solution A, add the above powder Prepare the soft material in the middle, pass through a 20-mesh sieve to make granules, dry at 40-60°C, sieve the 18-mesh sieve, add magnesium stearate to the dry granules, mix well, and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com