Preparation method of double-protected lysine containing boc side chain amino protection
A lysine, double protection technology, applied in the field of synthesis of double protected lysine (Fmoc-Lys-OH), can solve the problems of high price and high raw material cost, reduce costs, solve safety and environmental protection problems, solve The effect of high cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
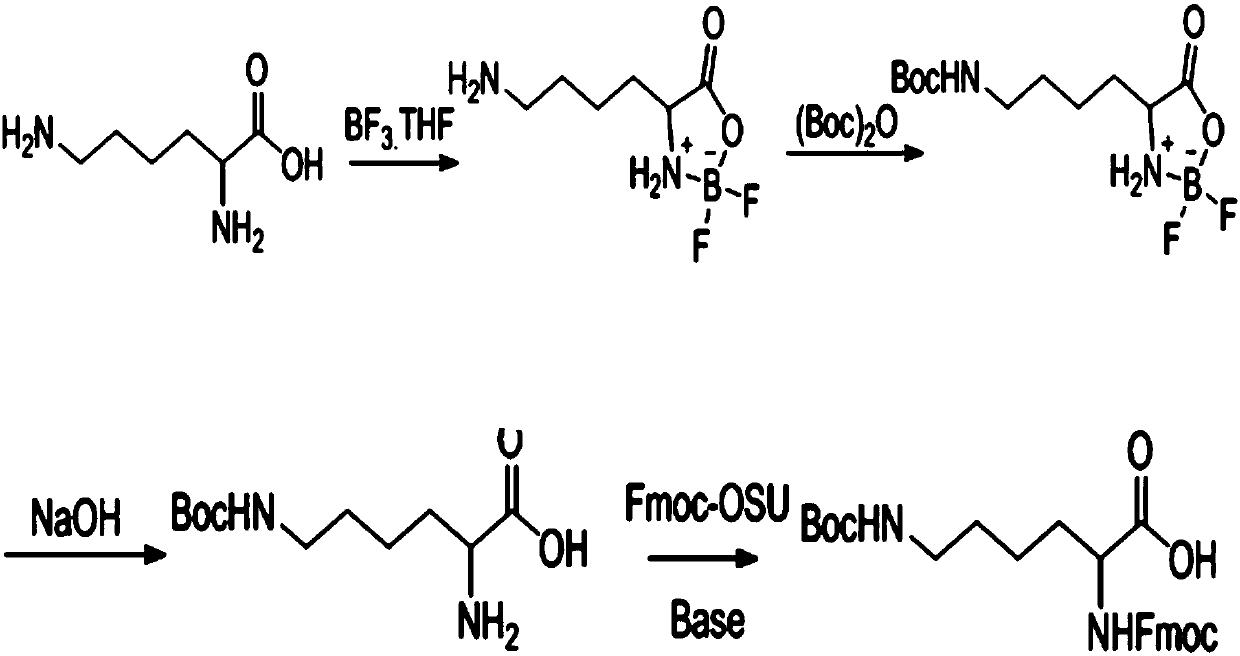
[0044] Example 1, the synthesis process of double-protected lysine (Fmoc-Lys(Boc)-OH) containing Boc side chain amino protection. refer to figure 1 .
[0045] ⑴. Disperse 146 grams of lysine in 300 ml of tetrahydrofuran; ⑵. Add 57 grams of sodium methoxide while stirring in an ice bath; ⑶. Naturally warm to room temperature and stir for 1 hour. ⑷. Add 203 grams of 50% boron trifluoride tetrahydrofuran solution; ⑸. Slowly heat up to 50 degrees; ⑹. Stir for 5 hours until the system is clear. ⑺. Add 262 grams of di-tert-butyl dicarbonate in tetrahydrofuran; ⑻. Control the pH of liquid caustic soda to about 8 until the reaction is complete. ⑼. Add 20 ml of liquid caustic soda; ⑽. Stir in ice bath for half an hour. ⑾. Tetrahydrofuran was recovered under reduced pressure, and a large amount of white solids were precipitated; ⑿. The wet intermediate H-Lys(Boc)-OH was obtained by centrifugation.
Embodiment 2
[0047] .Add 800 grams of water and 800 grams of ethyl acetate to the wet product of H-Lys(Boc)-OH obtained in Example 1, stir well and adjust the pH to 9 with liquid caustic soda. .Add about 350 grams of Fmoc-osu in batches until the reaction is complete, and use liquid caustic soda to control the pH to 9-10 during the reaction. .After the reaction, adjust the pH to 3 with industrial hydrochloric acid; . Separate the ethyl acetate layer, and wash the ethyl acetate layer with saturated brine; .Dried over anhydrous sodium sulfate, filtered with suction, and precipitated; . Add 4.3KG of tertiary methyl ether and beat for 2 hours, filter with suction, and dry the solid to obtain 202 grams. HPLC analysis product content is greater than 99.5%. . Suction filtration, and the solid was air-dried to obtain a product content greater than 99.5% according to HPLC analysis.
Embodiment 3
[0048] Embodiment 3 is basically the same as Embodiment 1 and 2, but has the following changes:
[0049] In step (1), the weight-volume ratio of lysine and THF is: 1:5;
[0050] In step (2), the mol ratio of lysine and sodium methylate is: 1:0.8;
[0051] In step (4), the mol ratio of lysine and boron trifluoride is: 1:0.9;
[0052] In step (7), the mol ratio of lysine and di-tert-butyl dicarbonate is: 1:0.9;
[0053] In step ⑼, the weight ratio of lysine to 30% liquid caustic soda is: 4:1; described liquid caustic soda adopts sodium hydroxide, potassium hydroxide, sodium carbonate or potassium carbonate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com