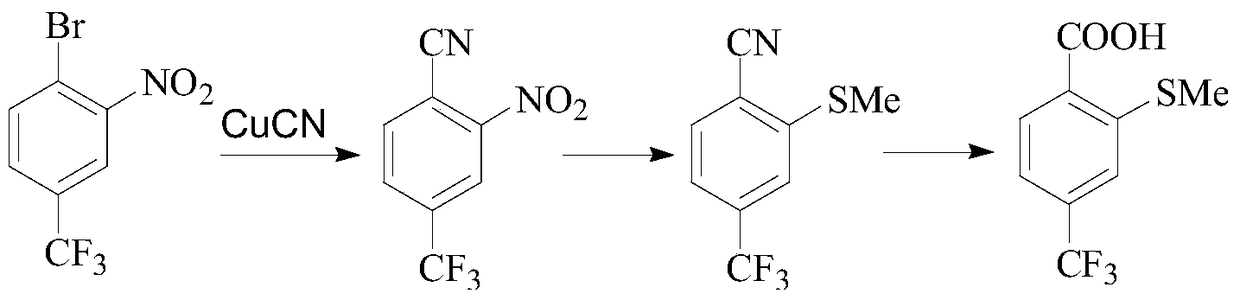
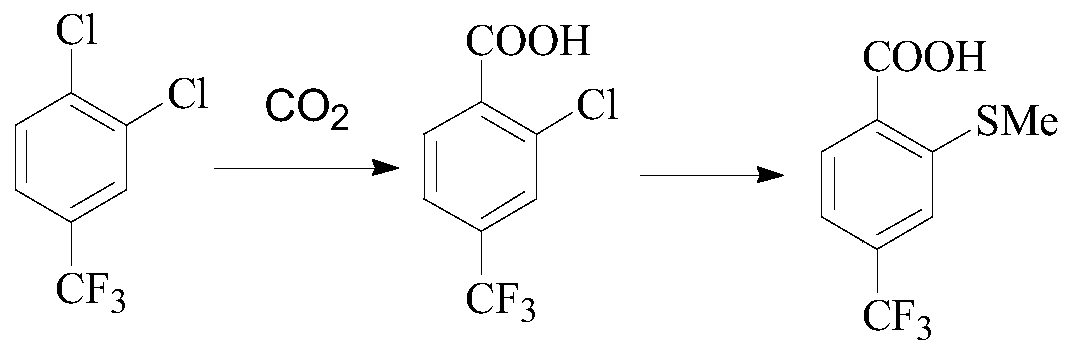
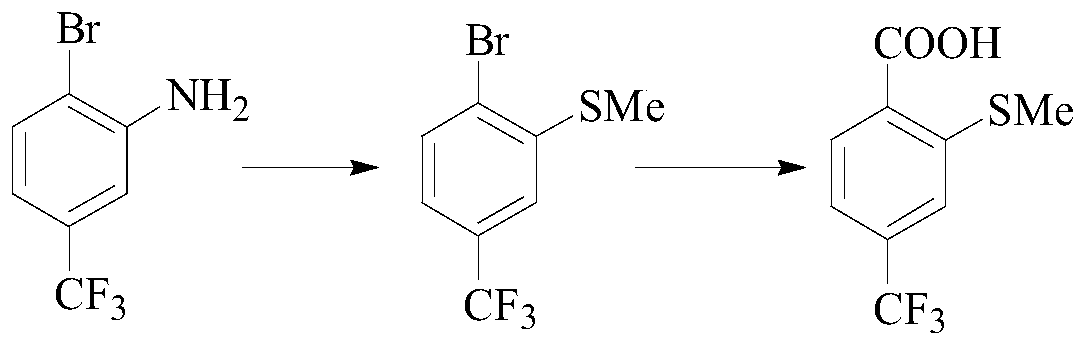
A kind of preparation method of sulfophenylpyrazolone and its intermediate
A technology of sulfophenylpyrazolone and alkylating reagent, which is applied in the field of organic synthesis, can solve the problems of unsuitability for industrial production, harsh reaction conditions, and high cost of raw materials, so as to avoid the problems of safety and three wastes, high total yield, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] A preparation method of sulfophenylpyrazolone, comprising the steps of:
[0042] Step 1, in a solvent and in the presence of a base, after compound (I) and compound (II) react at a certain temperature, continue to react with an oxidant to obtain compound (III);
[0043] Step 2, after compound (III) reacts with an acid chloride reagent, then reacts with alcohol at a certain temperature to obtain compound (IV), or compound (III) reacts with an alkylating agent at a certain temperature under the action of a base to obtain compound ( IV);
[0044] Step 3, react compound (IV) with sulfide (V) at a certain temperature in a solvent and in the presence of a base to obtain compound (VI);
[0045] Step 4, compound (VI) is hydrolyzed with alkali, then oxidized with hydrogen peroxide to form compound (VII), or oxidized with hydrogen peroxide, and then hydrolyzed with alkali to form compound (VII);
[0046] Step 5, compound (VII) is reacted with an acid chloride reagent to obtain ...
Embodiment 1
[0065] Embodiment 1: the preparation of 4-chloro-3-nitrobenzotrifluoride
[0066] In a 1000mL three-necked flask, add 400g of p-chlorobenzotrifluoride, add dropwise a mixed acid of 273g of 98% sulfuric acid and 190g of 98% nitric acid at 30°C, continue to react for 4 hours after the dropwise addition, separate the organic layer, and wash the organic layer with water Two times, 480g of 4-chloro-3-nitrobenzotrifluoride was obtained, the yield was 96%.
Embodiment 2
[0067] Embodiment 2: Preparation of 2-nitro-4-trifluoromethylbenzoic acid
[0068] In a 1000mL three-necked flask, add 500g DMF, 180g potassium carbonate, 78g ethyl cyanoacetate and 150g 4-chloro-3-nitrobenzotrifluoride, control the temperature of the reactant within 50°C, and stir for 1 hour. Then add 230g 35% H 2 o 2 , reacted for 2 hours, quenched hydrogen peroxide, distilled and recovered DMF, added water, acidified with hydrochloric acid, solid precipitated, filtered, washed the filter cake with water, and dried to obtain 149g of 2-nitro-4-trifluoromethylbenzoic acid. 1 H-NMRδppm (DMSO-d 6 ) 14.39 (br, 1H), 8.46 (s, 1H), 8.21 (d, J=8.0Hz, 1H), 8.09 (d, J=8.0Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com