One-step synthesized nitrogen and sulfur co-doped graphene aerosol and electro-adsorption removal of various heavy metal ions thereby
A technology of graphene airgel and nitrogen-sulfur co-doping, applied in separation methods, dispersed particle separation, chemical instruments and methods, etc., to achieve the effects of improved electrosorption performance, short time, and simple and easy preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation of nitrogen-sulfur co-doped graphene airgel paper electrode includes the following steps:
[0021] (1) Ultrasonic disperse 0.15g of graphene oxide (GO) in 100mL of distilled water, then add 0.45g of L-cysteine, stir it mechanically to make it fully mixed, and then put the mixture at 95°C Heated in a water bath for 2h. After the reaction, the resulting product was immersed in distilled water for 2-3 days, and finally the sample was freeze-dried at -52°C for 24 hours to obtain nitrogen-sulfur co-doped graphene airgel.
[0022] (2) Add 90mg of the nitrogen-sulfur co-doped graphene airgel material prepared in step (1) into 2mL of 4wt% polyvinyl alcohol solution, and ultrasonically disperse the composite material in the solution evenly. Take 0.16mL of the above dispersion liquid and apply it evenly on a 35mm×8mm hard paper sheet (thickness 400μm), and freeze-dry it at -52°C for 12h to make a nitrogen-sulfur co-doped graphene airgel paper electrode.
Embodiment 2
[0024] The preparation process of the nitrogen-sulfur co-doped graphene airgel paper electrode is the same as that of Example 1.
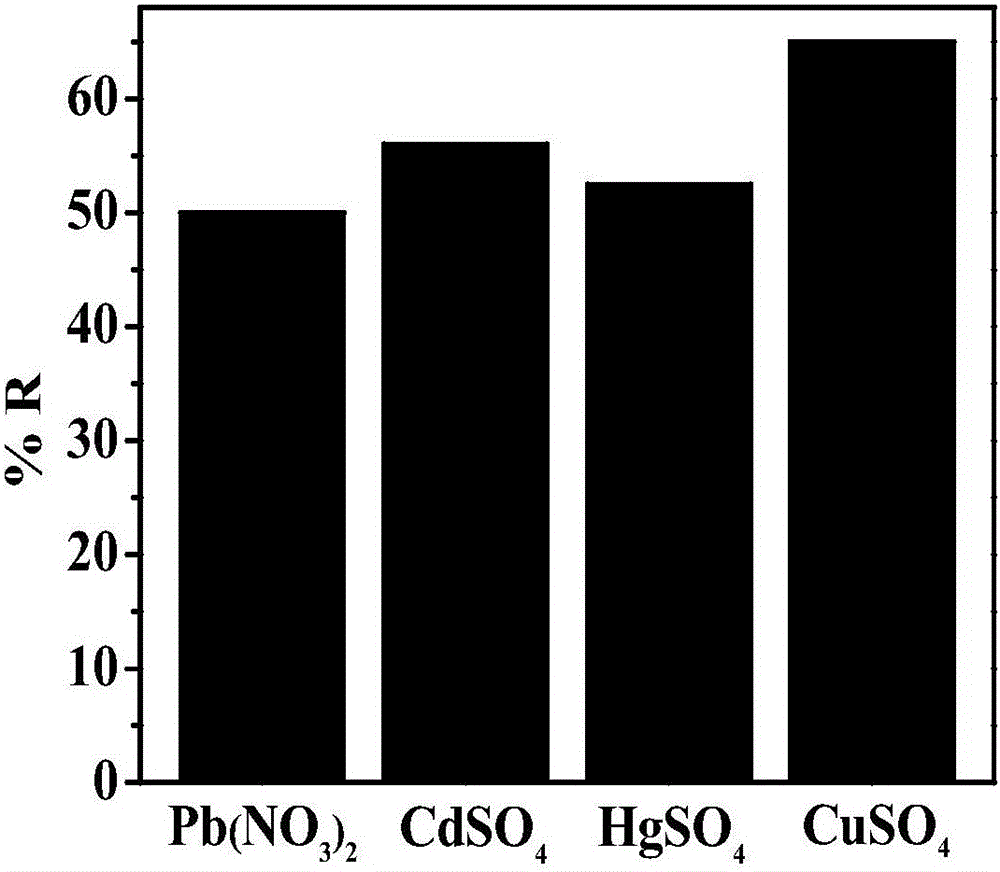
[0025] The prepared nitrogen-sulfur co-doped graphene airgel paper electrodes were used for Pb(NO 3 ) 2 , CdSO 4 , HgSO 4 and CuSO 4 Electrochemical treatment of the solution, the applied voltage is -0.3V, the treatment time is 2min, Pb 2+ 、Cd 2+ , Hg 2+ and Cu 2+ The removal rate see figure 2 , it can be seen that nitrogen and sulfur co-doped graphene airgel materials have a strong effect on heavy metal ions Cu 2+ The adsorption effect is the best.
Embodiment 3
[0027] The preparation process of the nitrogen-sulfur co-doped graphene airgel paper electrode is the same as that of Example 1.
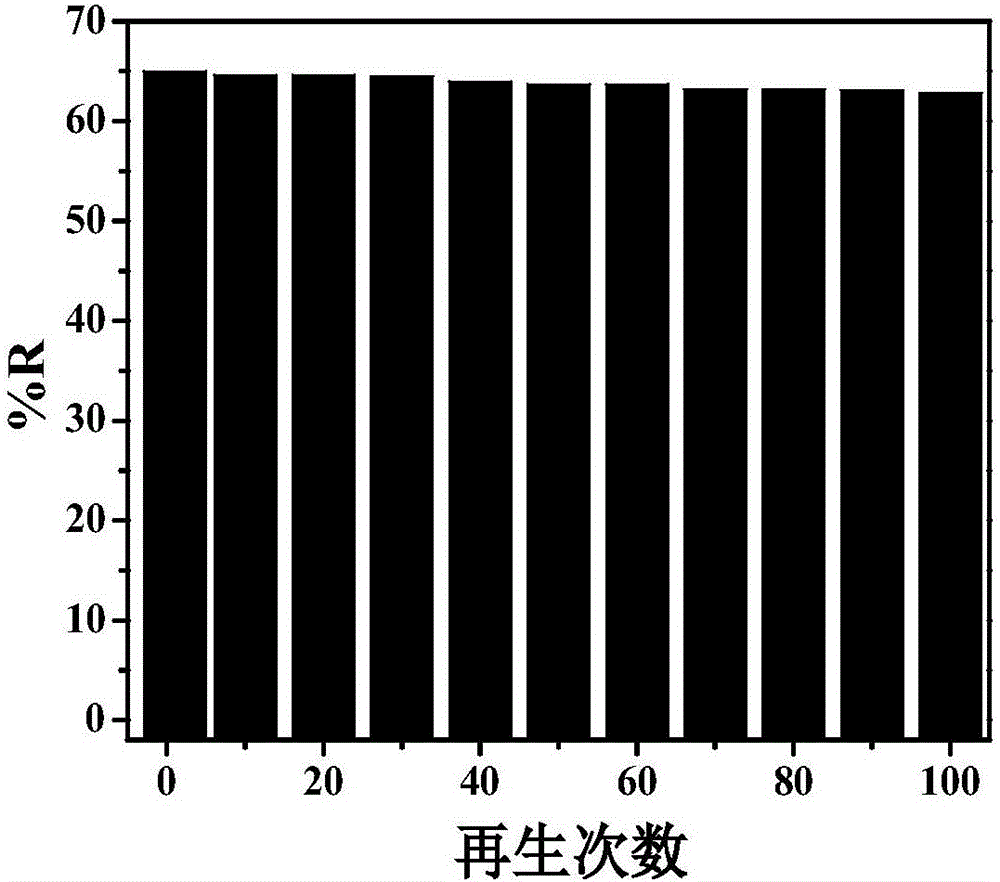
[0028] Cyclic electrosorption experiments on nitrogen-sulfur co-doped graphene airgel paper electrodes. to Cu 2+ As an example, a cyclic electrosorption experiment was carried out on a nitrogen-sulfur co-doped graphene airgel paper electrode. The nitrogen-sulfur co-doped graphene airgel paper electrode was placed in 80mL of CuSO with a concentration of 1mmol / L 4 In the solution, apply a potential of -0.3V, and record the conductivity of the solution, record the conductivity of the solution again after 2 minutes, and calculate the removal rate. Then the potential was removed to allow it to desorb, and the cycle was repeated several times. Experimental results such as image 3 shown. Adsorbed Cu for the first time 2+ The removal rate is 65%, and after 100 cycles of use, the electrode is on Cu 2+ The removal rate was 62%. It shows that the mat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com