Preparation method of sateripol compounds
The technology of a compound and gentisol, which is applied in the field of preparation of gentisol compounds, can solve the problems of high price, unfavorable mass production, and high raw material cost, and achieves the effects of ideal yield, reduced cost and easy implementation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
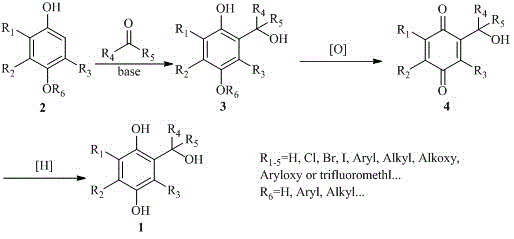
[0016] Embodiment 1: 2-hydroxymethyl-4-methoxyphenol ( 3 , see attached picture) for the synthesis of
[0017] Add 5 milliliters of deionized water, 4-methoxyphenol ( 2 , see attached image, follow references Organic & biomolecular chemistry 12.19(2014): 3100. Preparation, 2.0mmol), sodium metaborate tetrahydrate (16.0mmol), 0.75ml formalin solution, stirred and reacted at 40°C for 16 hours, TLC detected the end of the reaction. Add 1 ml of dilute hydrochloric acid to quench the reaction, extract the reaction solution with ethyl acetate (20mLx3), combine the organic phases, dry over anhydrous sodium sulfate, filter, concentrate, and purify by column chromatography to obtain 2-hydroxymethyl-4-methoxy Base phenol, the yield is 91%.
Embodiment 2
[0018] Example 2: Gentioquinone (2-hydroxymethyl-1,4-p-benzoquinone, 4 , see attached picture) for the synthesis of
[0019] Add 2 milliliters of acetonitrile, 2 milliliters of water, 2-hydroxymethyl-4-methoxyphenol ( 3 , see accompanying drawing, 1.0mmol), cerium ammonium nitrate (2.5mmol), stirred and reacted at 0°C for 2 hours, and TLC detected the end of the reaction. The reaction solution was extracted with dichloromethane (10mLx3), the organic phases were combined, dried over anhydrous sodium sulfate, filtered, concentrated, and purified by column chromatography to obtain gentioquinone with a yield of 87%. H NMR spectrum 1 HNMR (400MHz, CDCl 3 )σ6.79(s,1H),6.72(s,2H),4.51(s,2H); C NMR spectrum 13 CNMR (100MHz, CDCl 3 )σ 187.7, 187.5, 147.4, 136.7, 136.5, 131.1, 59.1.
Embodiment 3
[0020] Embodiment 3: Gentianol (2-hydroxymethyl-1,4-hydroquinone, 1 , see attached picture) for the synthesis of
[0021] Add 0.5 milliliters of dichloromethane, 1.5 milliliters of water, 2-hydroxymethyl-1,4-p-benzoquinone ( 4 , see attached figure, 0.2mmol), sodium dithionite (0.6mmol), stirred and reacted at room temperature for 2 hours, and TLC detected the end of the reaction. Add 1 ml of dilute hydrochloric acid to quench the reaction, separate the liquids after shaking, extract the aqueous phase with an organic solvent (10mLx3), combine the organic phases, dry over anhydrous sodium sulfate, filter, concentrate, and purify by column chromatography to obtain gentianol. The yield is 80%. H NMR spectrum 1 HNMR (400MHz, DMSO-d 6 )σ6.72(s,1H),6.54(d,1H,J=8.4Hz),6.41(d,1H,J=8.4Hz),4.39(s,2H); C NMR spectrum 13 CNMR (100MHz, DMSO-d 6 )σ150.2, 146.8, 129.8, 115.5, 114.4, 113.7, 58.7; high resolution mass spectrometry HRMS (TOFMSES + ) m / z :CalcdforC 7 h 8 o 3 [M + ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com