A kind of synthetic method of austrodoral and austrodoric acid
A technology for compounds and natural products, applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve the problems of expensive starting materials or reagents, cumbersome experimental operations, long synthetic routes, etc. Ease of implementation, easy reaction conditions, and desirable yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
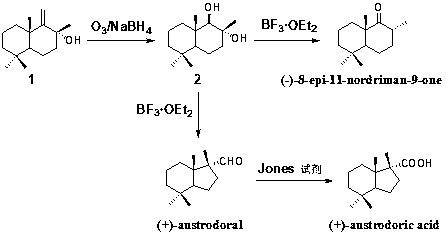
[0019] Embodiment 1: Synthesis of sesquiterpene vicinal diol compound (2, see accompanying drawing).
[0020] At -78°C, first 444mg of sesquiterpene alcohol compound (1, see accompanying drawing, 2.0mmol) was dissolved in 10mL of dichloromethane, and ozone was passed into the reaction system until it turned blue; where N 2 Exhaust the ozone remaining in the reaction system from the reaction system; finally add NaBH to the reaction system 4 (6.0mmol), slowly warming up to room temperature, stirring for 2-3 hours; TLC detection of the end of the reaction, adding 5mL dilute hydrochloric acid to quench the reaction, the reaction solution was extracted with dichloromethane (15mL x 3), combined organic phase, anhydrous sodium sulfate Dry, filter, concentrate, and purify by column chromatography to obtain 452 mg of white solid with a yield of 100%.
Embodiment 2
[0021] Example 2: Synthesis of marine terpenoid natural product austrodoral (see accompanying drawing).
[0022] Take 226mg of sesquiterpene o-diol compound (2, 1.0mmol) and dissolve it in 5mL of dichloromethane, add 0.1mmol of boron trifluoride ether, stir and react at room temperature for 12 hours, TLC detects that the reaction is complete, and then add 10mL of saturated ammonium chloride The solution quenched the reaction, and the reaction solution was extracted with dichloromethane (15mL x 3), the organic phases were combined, dried over anhydrous sodium sulfate, filtered, concentrated, and purified by column chromatography to obtain 177 mg of a colorless oily substance austrodoral, with a yield of 85% . H NMR spectrum 1 H-NMR (400MHz, CDCl 3 )δ:0.83(3H,s),0.85(3H,s),0.86(3H,s),0.94(1H,ddd,J=4.0,13.0,13.0Hz),1.01(3H,s),1.16(1H ,dt,J=4.0,13.0Hz),1.22(1H,dd,J=7.5,12.5Hz),1.27(1H,ddd,J=2.5,11.0,13.0Hz),1.33–1.51(3H,m), 1.52–1.70(3H,m),2.13(1H,ddd,J=6.0,9.5,13.0Hz),9.65(1H...
Embodiment 3
[0023] Example 3: Synthesis of terrestrial terpenoid natural product 8-epi-11-nordriman-9-one (see attached figure).
[0024] Take 226 mg of sesquiterpene vicinal diol compound (2, see attached figure, 1.0 mmol) and dissolve it in 5 mL of dichloromethane, add 2.0 mmol of boron trifluoride ether, stir and react at room temperature for 2 hours, and TLC detects that the reaction is complete. Add 10mL of saturated ammonium chloride solution to quench the reaction, the reaction solution was extracted with dichloromethane (15mL x 3), the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated, and purified by column chromatography to give Color solid 8-epi-11-nordriman-9-one 198 mg, yield 95%. H NMR spectrum 1 H NMR (400MHz, CDCl 3 )δ: 2.68(m, 1H), 2.10(ddd, 1H, J=3.2Hz, J=6.3Hz, J=14.0Hz), 1.72(m, 1H), 1.57(m, 4H), 1.40(d, 1H,J=13.5Hz),1.23(m,3H),1.14(s,3H),1.09(m,1H),0.98(d,3H,J=6.4Hz),0.93(s,3H),0.88( s,3H); C NMR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com