A kind of synthetic method of methoxyphenamine hydrochloride
A technology of methoxamine hydrochloride and a synthesis method, which is applied in the field of synthesis of pharmaceutical raw materials and chemical intermediates, can solve the problems of easy explosion, high risk, increased production cost, etc., and achieves avoiding explosion, mild reaction conditions, and reducing reaction time. cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
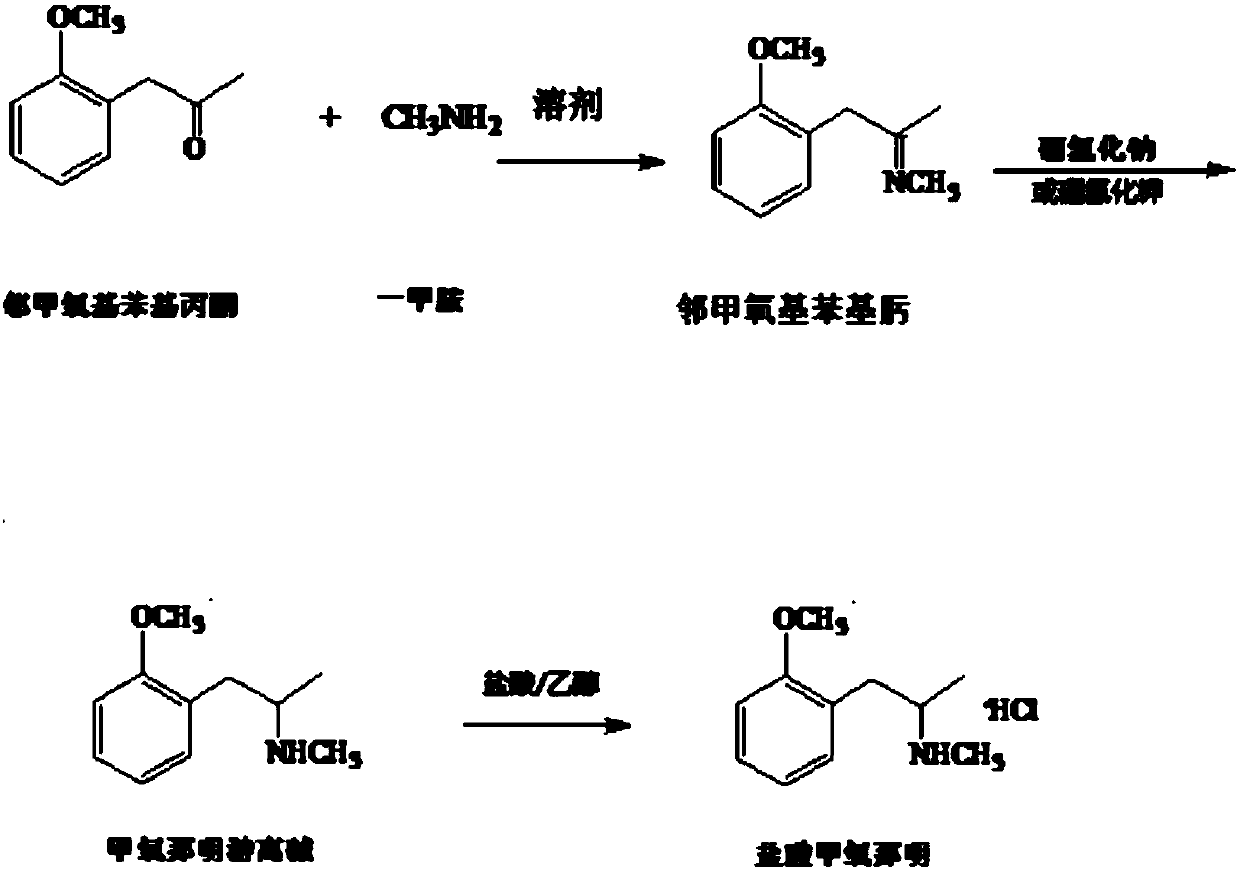
[0023] Add 80kg of o-methoxyphenylacetone and 240g of methanol into the reactor, stir and mix at 25°C for 30 minutes, pass through methylamine gas, control the temperature at 20°C, and ventilate for about 11 hours, then react to o-methoxy If the concentration of phenylacetone is lower than 5% of the reaction solution, stop the aeration.
[0024] Cool down to -8°C, add 14.4kg of sodium borohydride in portions, stir for 1 hour, add 50kg of water to destroy unreacted sodium borohydride; vacuum distill to remove the solvent, then add 100kg of water, stir for 1.5 hours, and let stand for 1 hour. The water separator was opened to separate the water layer, and the water was distilled off by vacuum distillation.
[0025] Add 64kg of absolute ethanol, stir for 1 hour, filter, cool down to below 0°C, add hydrochloric acid / ethanol solution dropwise to the filtrate to adjust PH=2; at -8°C, crystallize for 12 hours, and filter to obtain crude methoxyphenamine hydrochloride , after recryst...
Embodiment 2
[0027] Add 80kg of o-methoxyphenylacetone and 280kg of methanol into the reactor, stir and mix at 25°C for 30 minutes, pass through methylamine gas, control the temperature at 35°C, and ventilate for about 13 hours, then react to o-methoxy If the concentration of phenylacetone is lower than 5% of the reaction solution, stop the aeration.
[0028] Cool down to -10°C, add 16kg of sodium borohydride in portions, stir for 1 hour, add 50kg of water to destroy unreacted sodium borohydride; remove the solvent by vacuum distillation, then add 100kg of water, stir for 1.5 hours, stand for 1 hour, open The water layer is separated by a water separator, and the water is evaporated by vacuum distillation.
[0029] Add 80 kg of absolute ethanol, stir for 1 hour, filter, cool down to below 0°C, add hydrochloric acid / ethanol solution dropwise to the filtrate to adjust PH=3; at -8°C, crystallize for 12 hours, and filter to obtain crude methoxyphenamine hydrochloride , after recrystallization...
Embodiment 3
[0031] Add 80kg of o-methoxyphenylacetone and 264kg of methanol into the reactor, stir and mix at 25°C for 30 minutes, pass through methylamine gas, control the temperature at 30°C, and ventilate for about 12 hours, then react to o-methoxy If the concentration of phenylacetone is lower than 5% of the reaction solution, stop the aeration.
[0032] Cool down to -9°C, add 15.2kg of sodium borohydride in portions, stir for 1 hour, add 50kg of water to destroy unreacted sodium borohydride; vacuum distillation to remove the solvent, then add 100kg of water, stir for 1.5 hours, and let stand for 1 hour. The water separator was opened to separate the water layer, and the water was distilled off by vacuum distillation.
[0033] Add 72 kg of absolute ethanol, stir for 1 hour, filter, cool down to below 0°C, add hydrochloric acid / ethanol solution dropwise to the filtrate to adjust PH = 2; at -8°C, crystallize for 12 hours, filter to obtain crude methoxyphenamine hydrochloride , after re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com