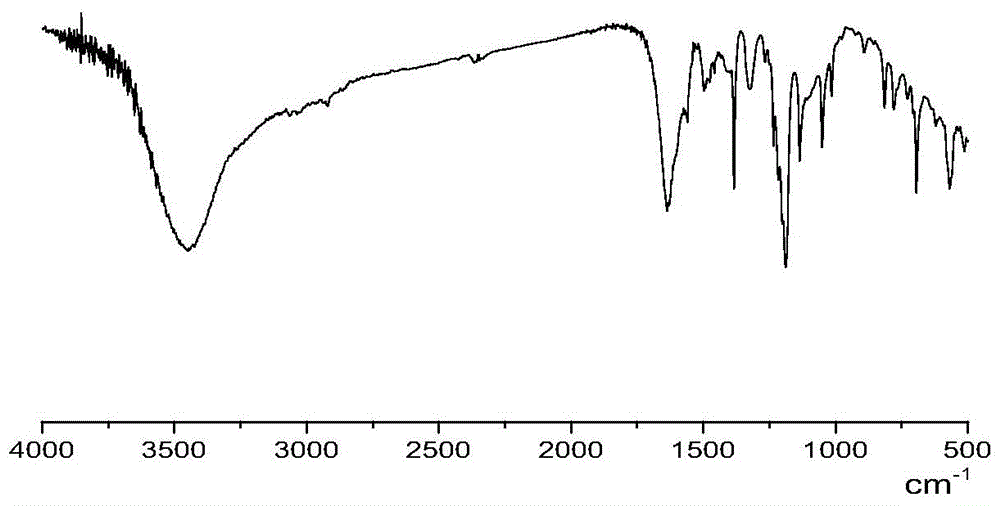
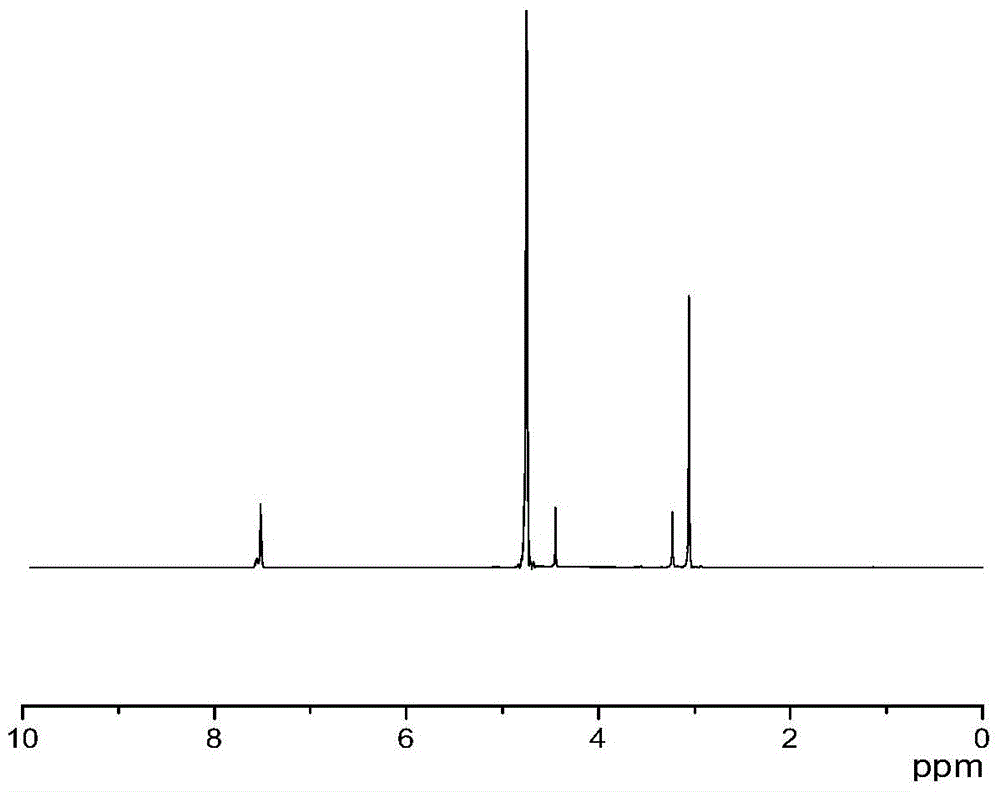
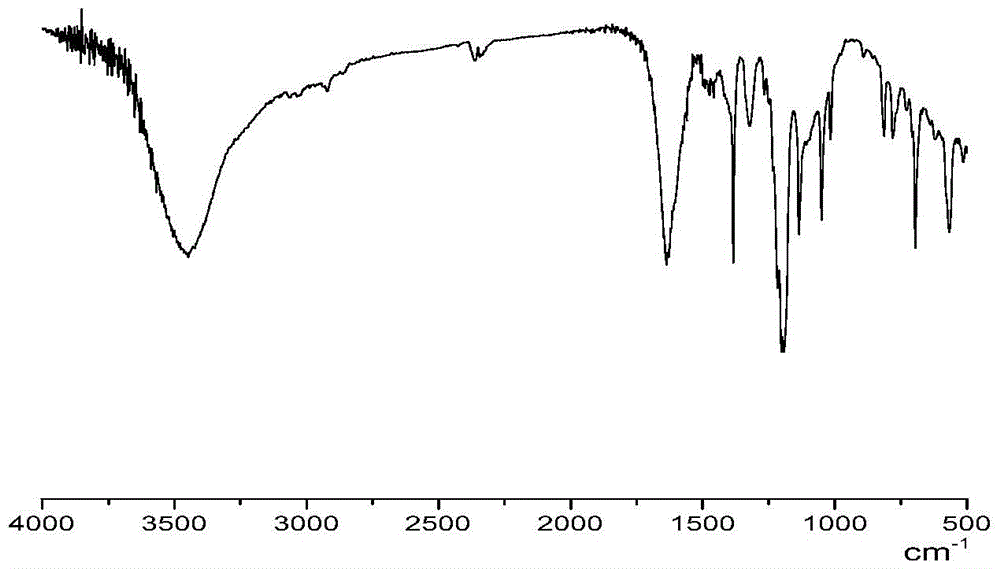
Amino acid ionic liquid molecules and preparation method and application thereof
A technology for ionic liquids and amino acids, which is applied in the field of preparation of amino acid ionic liquids to achieve the effects of reduced energy consumption, mild reaction conditions, and low environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Transfer a certain amount of C into a 250mL three-neck flask 1 ~C 20 alkane amine (water or alcohol) solution, and then drop propylene oxide (propylene oxide and alkane amine are added in equimolar amounts) with a dropping funnel, and the dropwise addition is completed in about 30 minutes. React for 2-8 hours. After the reaction is over, raise the temperature of the product mixture to 35°C, and steam the unreacted propylene oxide to obtain the tertiary aminated product solution. Add an equimolar amount of halogenated hydrocarbon according to the amount of the tertiary aminated product. (such as benzyl chloride), stir the reaction at constant temperature (40-80°C) for a certain period of time, and end the reaction. The product mixture is layered, and the lower layer is unreacted benzyl chloride. Remove traces of benzyl chloride that may remain in the water layer, remove the ether layer, and vacuum-dry the resulting solution to obtain the quaternary ammonium salt product...
Embodiment 2
[0041] Transfer a certain amount of C into a 250mL three-neck flask 1 ~C 15 alkane amine (water or alcohol) solution, then drop propylene oxide (propylene oxide and alkane amine in 2:1 molar amount) with a dropping funnel, and drop it for about 30 minutes, keep stirring at low temperature (0-5°C) The reaction was continued for 2-8 hours. After the reaction, the temperature of the product mixture was raised to 35°C, and the unreacted propylene oxide was evaporated to obtain the tertiary aminated product solution. Equimolar amounts of halogen were added according to the amount of the tertiary aminated product. After substituting a hydrocarbon (such as benzyl chloride), stir the reaction at a constant temperature (40-80°C) for a certain period of time, and end the reaction. The product mixture is layered, and the lower layer is unreacted benzyl chloride. After separation, the product solution is extracted twice with ether , to remove traces of benzyl chloride that may remain in ...
Embodiment 3
[0045] Transfer a certain amount of C into a 250mL three-neck flask 1 ~C 15 alkane amine (water or alcohol) solution, then drop propylene oxide (propylene oxide and alkane amine in 3:1 molar amount) with a dropping funnel, and drop it for about 30 minutes, keep stirring at low temperature (0-5°C) The reaction was continued for 2-8 hours. After the reaction, the temperature of the product mixture was raised to 35°C, and the unreacted propylene oxide was evaporated to obtain the tertiary aminated product solution. Equimolar amounts of halogen were added according to the amount of the tertiary aminated product. After substituting a hydrocarbon (such as benzyl chloride), stir the reaction at a constant temperature (40-80°C) for a certain period of time, and end the reaction. The product mixture is layered, and the lower layer is unreacted benzyl chloride. After separation, the product solution is extracted twice with ether , to remove traces of benzyl chloride that may remain in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com