Preparation method of polysubstituted thiapyran diindyl derivative
A technology of indole derivatives and multi-substitution is applied in the field of preparation of multi-substituted thiopyranoindole derivatives, which can solve the problems of difficulty in synthesizing multi-substituted thiopyranoindole derivatives, expensive catalytic metals, and many synthesis steps. , to achieve the effect of easy purification of the product, simple synthesis method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
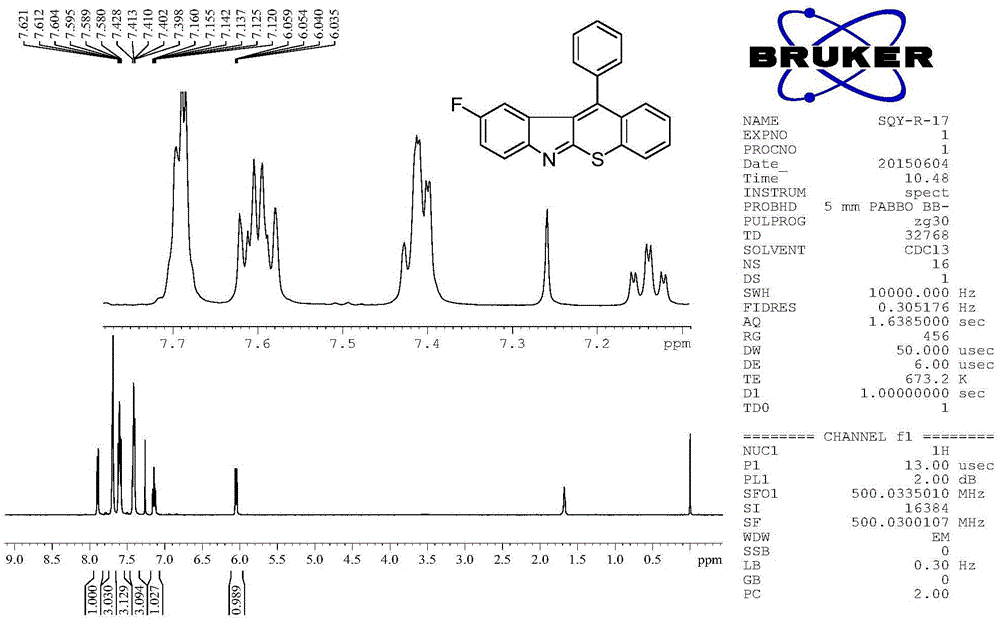
Examples
Embodiment 1
[0034] Example 1: 11-phenylbenzothiopyranoindole (R in structural formula I 9 = phenyl)
[0035] Add o-alkynyl isothiocyanate (0.5mmol, 117mg), diaryl hyperiodonium salt (0.75mmol, 313mg) and copper salt (0.05mmol), potassium carbonate (70mg, 0.5mmol) to a 10mL Shrek tube , after replacing the nitrogen gas three times, 2.5 mL of dichloroethane was added and reacted at 50° C. for 4 h. After the reaction system was cooled, 5 mL of saturated NaHCO was added 3 The solution quenched the reaction, and then extracted three times with 30 mL ethyl acetate, combined the organic phases, washed with saturated NaCl and washed with anhydrous MgSO 4 Dry for 30 minutes, filter, and concentrate the filtrate with a rotary evaporator to obtain a crude product. The crude product was separated by column chromatography (200-300 mesh silica gel) to obtain 100 mg of the red solid product 11-phenylthiopyranoindole with a purity greater than 99%, and the isolated yield was 64%.
[0036] Structural ...
Embodiment 2
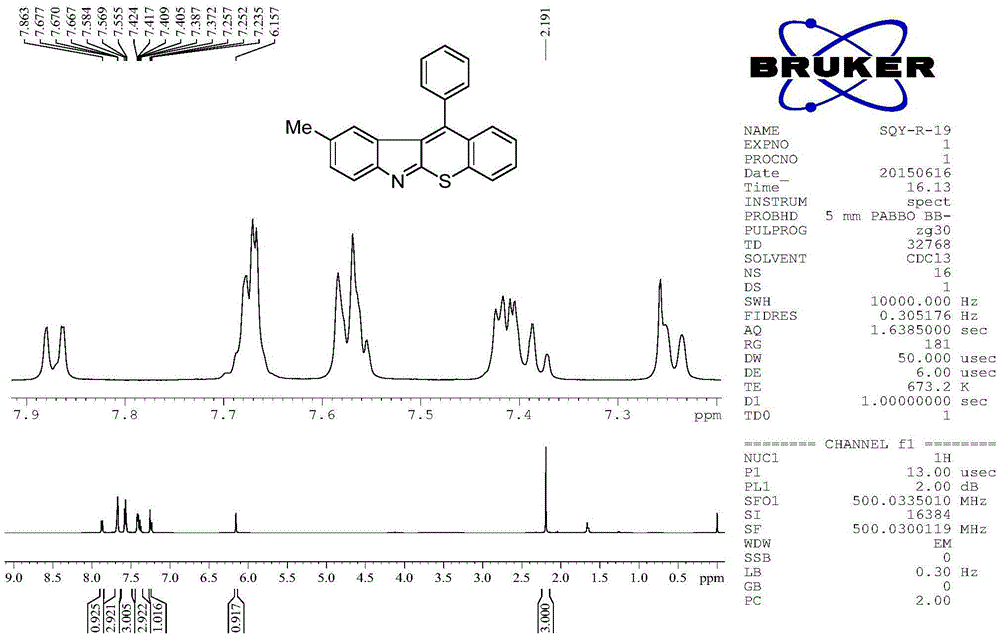
[0041] Embodiment 2: 3-methyl-11-phenylbenzothiopyranoindole (R in structural formula I 6 = methyl, R 9 = phenyl)
[0042] Add o-alkynyl isothiocyanate (0.5mmol, 124mg), diaryl hyperiodonium salt (0.75mmol, 313mg) and copper salt (0.05mmol), potassium carbonate (70mg, 0.5mmol) to a 10mL Shrek tube , after replacing the nitrogen gas three times, 2.5 mL of dichloroethane was added and reacted at 50° C. for 4 h. After the reaction system was cooled, 5 mL of saturated NaHCO was added 3 The solution quenched the reaction, and then extracted three times with 30 mL ethyl acetate, combined the organic phases, washed with saturated NaCl and washed with anhydrous MgSO 4 Dry for 30 minutes, filter, and concentrate the filtrate with a rotary evaporator to obtain a crude product. The crude product was separated by column chromatography (200-300 mesh silica gel) to obtain 99 mg of 3-methyl-11-phenylbenzothiopyranoindole as a red solid product with a purity of more than 99%, and the isol...
Embodiment 3
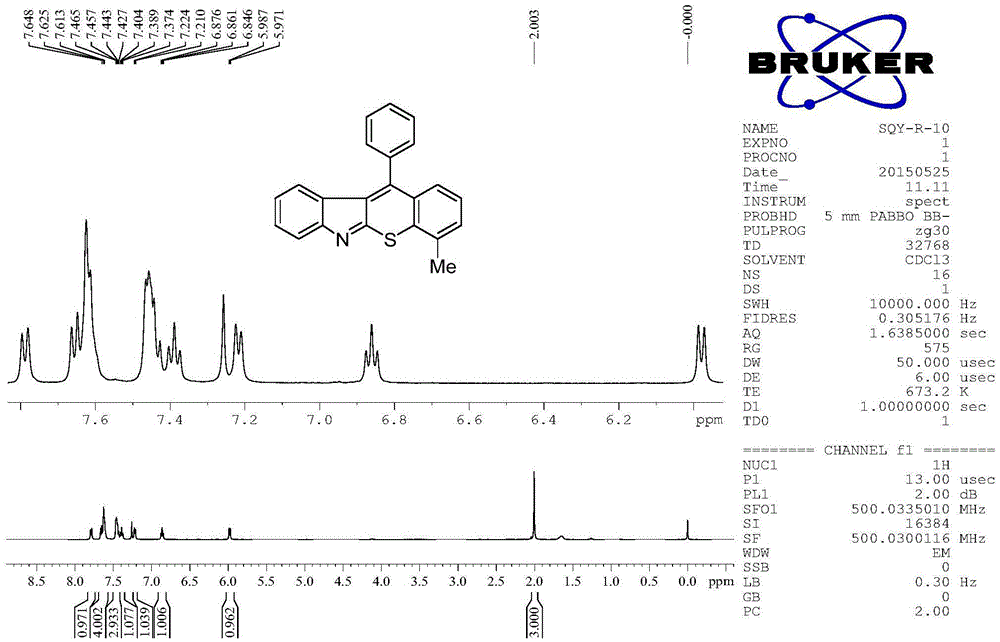
[0048] Embodiment 3: 2-methyl-11-phenylbenzothiopyranoindole (R in structural formula I 7 = methyl, R 9 = phenyl)
[0049] Add o-alkynyl isothiocyanate (0.5mmol, 124mg), diaryl hyperiodonium salt (0.75mmol, 313mg) and copper salt (0.05mmol), potassium carbonate (70mg, 0.5mmol) to a 10mL Shrek tube , after replacing the nitrogen gas three times, 2.5 mL of dichloroethane was added and reacted at 50° C. for 4 h. After the reaction system was cooled, 5 mL of saturated NaHCO was added 3 The solution quenched the reaction, and then extracted three times with 30 mL ethyl acetate, combined the organic phases, washed with saturated NaCl and washed with anhydrous MgSO 4 Dry for 30 minutes, filter, and concentrate the filtrate with a rotary evaporator to obtain a crude product. The crude product was separated by column chromatography (200-300 mesh silica gel) to obtain 100 mg of a red solid product 2-methyl-11-phenylbenzothiopyranoindole with a purity of more than 99%, and the isolat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com