Method for synthetizing carvacrol through enediol
A technology of enediol and carvacrol, applied in the field of synthesis of carvacrol from ? The process is simple and the synthesis process is green and environmentally friendly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
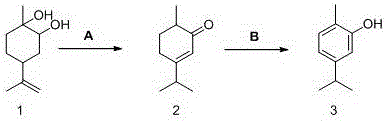
[0019] Step 1: Add 600g of diol, 300g of toluene and 18g of p-toluenesulfonic acid into a 1000mL reactor, then react at 120-150°C for about 3-4 hours, after the GC shows that the reaction is over, lower the temperature of the kettle to below 40°C , add 300g5wt%NaHCO 3 The aqueous solution was washed to pH=7-8, and the organic phase obtained by liquid separation was rectified under reduced pressure to obtain 510 g of isodihydrocarvone with a content greater than 90%, with a yield of 85%.
[0020] Step 2 Put 510g of isodihydrocarvone and 40g of xylene obtained above into a 1000mL three-necked flask, then add 400g of catalyst B, and react under reflux at 190-220°C. During the reaction, pump continuously to the reaction liquid level Blow air for about 4 to 5 hours, GC shows that 30% of isodihydrocarvone remains, stop the reaction, remove the catalyst by filtration, carry out rectification under reduced pressure on the filtrate, recover 37g of solvent and 151g of unreacted raw mate...
Embodiment 2
[0023] Step 1: Add 600g of diol, 300g of toluene and 15g of p-toluenesulfonic acid into a 1000mL reactor, then react at 120-150°C for about 4-5 hours, after the GC shows that the reaction is over, lower the temperature of the kettle to below 40°C , add 250g5wt%NaHCO 3 The aqueous solution was washed to PH = 7-8, and the organic phase obtained by liquid separation was rectified under reduced pressure to obtain 504 g of isodihydrocarvone with a content greater than 90%, with a yield of 84%.
[0024] Step 2 Put 504g of isodihydrocarvone and 35g of xylene obtained above into a 1000mL three-necked flask, then add 300g of catalyst B, and react under reflux at 190-220°C. During the reaction, pump continuously to the reaction liquid level Blow air for about 5-6 hours, GC shows that 38% of isodihydrocarvone remains, stop the reaction, remove the catalyst by filtration, carry out rectification under reduced pressure on the filtrate, recover 30g of solvent and 190g of unreacted raw mater...
Embodiment 3
[0026] Step 1: Add 600g of diol, 300g of toluene and 7g of p-toluenesulfonic acid into a 1000mL reactor, and then react at 120-150°C for 6-7 hours. After the GC shows that the reaction is over, lower the temperature of the kettle to below 40°C. Add 200g5wt%NaHCO 3 The aqueous solution was washed to pH=7-8, and the organic phase obtained by liquid separation was rectified under reduced pressure to obtain 486 g of isodihydrocarvone with a content greater than 90%, with a yield of 81%.
[0027] Step 2 Put 486g of isodihydrocarvone and 30g of xylene obtained above into a 1000mL three-necked flask, then add 150g of catalyst B, and react under reflux at 190-220°C. During the reaction, pump continuously to the reaction liquid level Blow the air for about 9 to 10 hours, GC shows that 34% of isodihydrocarvone remains, stop the reaction, remove the catalyst by filtration, carry out vacuum distillation on the filtrate, recover 28g of solvent and 164g of unreacted raw materials, and furth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com