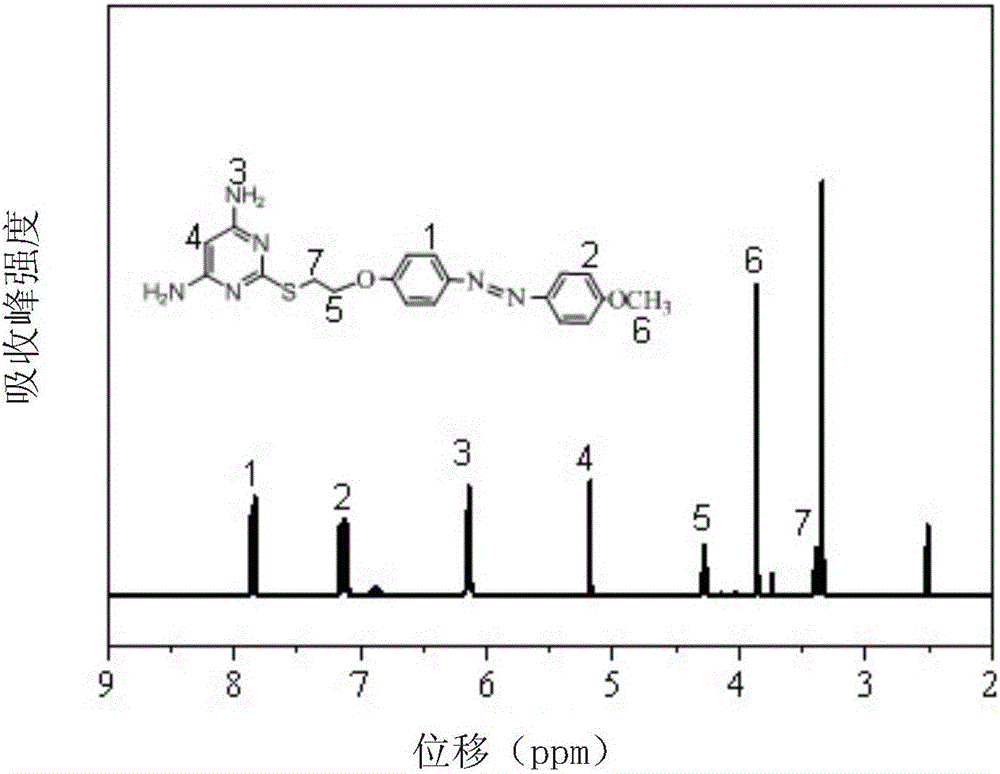
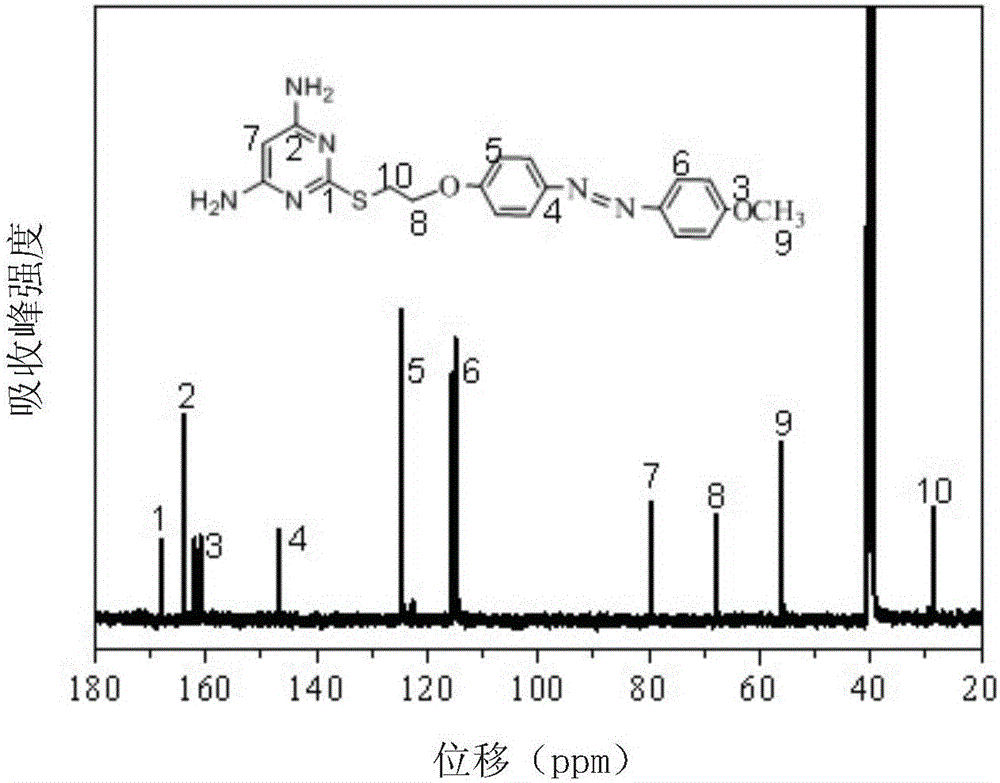
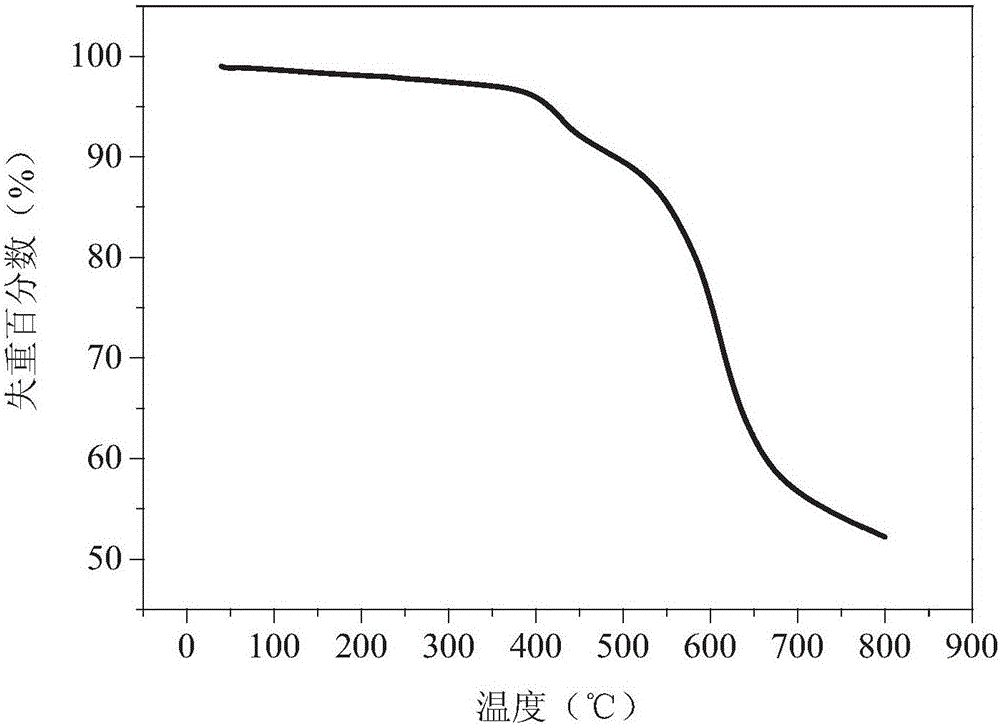
Method for preparing polyimide polymer with azo-pyrimidine structure unit
A technology of azopyrimidine and polyimide, which is applied in the field of polyimide preparation to achieve excellent thermal performance, increased light transmittance, and good photoalignment performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] A preparation method of a diamine monomer comprising an azopyrimidine structure, comprising the following steps:
[0043] 1) Synthesis of 4-hydroxy-4`-substituted azobenzene: Dissolve 4-substituted aniline in a solution containing concentrated HCl / H 2 O mixed solution (the volume ratio of concentrated HCl and water is 1:3-4) in a beaker, after it is completely dissolved, place it in an ice-water bath to cool to obtain a 4-substituted aniline acid solution. Under vigorous stirring, add cold sodium nitrite aqueous solution dropwise to the 4-substituted aniline acid solution, the molar ratio of sodium nitrite and 4-substituted aniline is 1:1, to obtain the diazonium salt solution of 4-substituted aniline, And keep the temperature of this solution below 5°C. In another beaker, dissolve phenol in 10% aqueous sodium hydroxide solution, cool the alkaline solution of phenol (sodium phenate) to below 5°C in an ice-water bath, and slowly add the diazonium salt solution of 4-subs...
Embodiment 1
[0065] This embodiment prepares polyimide containing ether linkages with azopyrimidine diamine monomers, and the structural formula is as follows:
[0066]
[0067] Among them, n is 20-80;
[0068] Obtained by the following steps: 1) Synthesis of 4-hydroxy-4`-methoxyazobenzene: 4-methoxyaniline (6.1625g, 50mmol) was dissolved in 50mL concentrated HCl / H2 O(V HCl :V H2O = 1:4) in the beaker of the mixed solution, after it was completely dissolved, it was placed in an ice-water bath and cooled to below 5°C. Under vigorous stirring, 10 mL of cold sodium nitrite (3.5237 g, 50 mmol) aqueous solution was added dropwise to this solution to obtain a diazonium salt solution of 4-methoxyaniline, and the diazonium salt of 4-methoxyaniline The solution was kept below 5°C. In another beaker, phenol (4.7012g, 50mmol) was dissolved in 25mL of 10% aqueous sodium hydroxide solution, cooled to below 5°C in an ice-water bath, and the diazonium salt solution of 4-methoxyaniline was slowly A...
Embodiment 2
[0071] This example prepares biphenyl-based polyimides with azopyrimidine-containing diamine monomers, and the structural formula is as follows:
[0072]
[0073] Among them, n is 20-80;
[0074] Obtained by the following steps: 1) Synthesis of 4-hydroxyl-4'-cyanoazobenzene: 4-cyanoaniline (5.9532g, 50mmol) was dissolved in 50mL concentrated HCl / H 2 O(V HCl :V H2O = 1:4) in the beaker of the mixed solution, after it was completely dissolved, it was placed in an ice-water bath and cooled to below 5°C. Under vigorous stirring, 10 mL of cold sodium nitrite (3.5237 g, 50 mmol) aqueous solution was added dropwise to this solution to obtain a diazonium salt solution of 4-cyanoaniline, and the diazonium salt solution of 4-cyanoaniline was kept Keep below 5°C. In another beaker, dissolve phenol (4.7012g, 50mmol) in 25mL of 10% aqueous sodium hydroxide solution, cool it to below 5°C in an ice-water bath, and slowly add the diazonium salt solution of 4-cyanoaniline dropwise , an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com