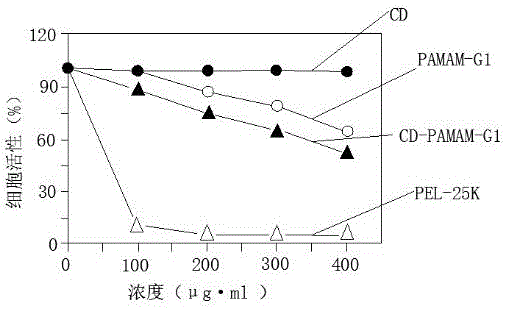
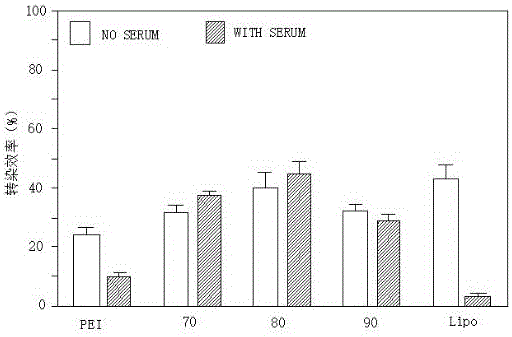
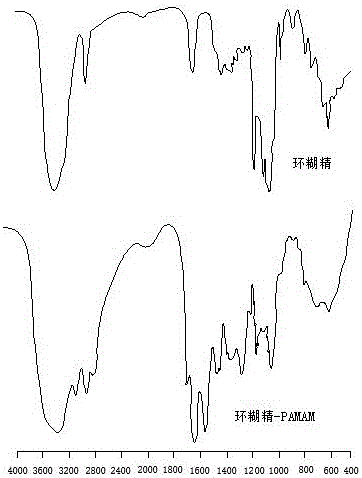Polyamide-amine (PAMAM)-based cyclodextrin functionalized derivative carrier system and application thereof
A carrier system, amine functionalization technology, applied in cardiovascular system diseases, non-active components of polymer compounds, medical preparations containing active components, etc., can solve the problems of high immunogenicity, vascular endothelial damage, poor compliance, etc. Achieving good application prospects and inhibiting the effect of tissue cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The preparation method of the polyamide-amine-based cyclodextrin functionalized derivative carrier system comprises the following steps:
[0031] (1) Quantitatively weigh cyclodextrin in a 500ml round bottom flask, add anhydrous DMSO to dissolve and form a clear and transparent solution A, quantitatively weigh N,N-carbonyldiimidazole, add anhydrous DMSO solution to form a clear solution B ;
[0032] (2) Under nitrogen protection conditions, add solution A dropwise to solution B at a rate of 5ml / h. After the dropwise addition, stir at room temperature for 16-24h under dark conditions, and add the reaction solution obtained after stirring to diethyl ether and In the mixed solution of ethyl acetate, centrifuge at 3000rpm for 10min at room temperature, discard the supernatant to obtain a white viscous precipitate, wash the white viscous precipitate with a mixture of ether and tetrahydrofuran for 2-3 times, and dry it under reduced pressure. An organic solvent is dissolved ...
Embodiment 1
[0040] Weigh 0.114g of β-CD, add 25ml of anhydrous DMSO to dissolve to form a clear solution A, and accurately weigh 0.811g of N,N-carbonyldiimidazole, place it in a 500ml round bottom flask, add 25ml of anhydrous DMSO to dissolve into solution B, Under nitrogen protection and rapid magnetic stirring, drop solution A into solution B at a rate of 1ml / 5min, and stir at room temperature in the dark for 16h; add the resulting product solution to a mixed solution of 900ml ether and ethyl acetate, resulting in a white Turbidity; centrifuge the turbid solution at 3000rpm for 10min, discard the supernatant solution and wash 2-3 times with the above-mentioned diethyl ether and ethyl acetate mixture, and set aside; use 25ml of anhydrous DMSO to dissolve the obtained solid in solution C, and then precisely Weigh PAMAM-G11.02g and dissolve in 150ml of anhydrous DMSO to obtain solution D; under the protection of nitrogen, slowly add solution C to solution D, and stir for 16 hours in the dar...
Embodiment 2
[0042] Weigh 0.224g of β-CD, add 25ml of anhydrous DMSO to dissolve to form a clear solution A, and accurately weigh 2.044g of N,N-carbonyldiimidazole, put it in a 500ml round bottom flask, add 25ml of anhydrous DMSO to dissolve into solution B; Under nitrogen protection and under the condition of rapid magnetic stirring, drop solution A into solution B at a rate of 1ml / 5min, and stir at room temperature for 16h in the dark; Turbid; centrifuge the turbid solution at 3000rpm for 10min, discard the supernatant solution and wash 2-3 times with the above mixed solution of diethyl ether and ethyl acetate, and set aside; use 25ml of anhydrous DMSO to dissolve the solid obtained in the previous step. , and then accurately weighed PAMAM-G110.224g and dissolved in 150ml of anhydrous DMSO to obtain solution D; under the protection of nitrogen, slowly added solution C to solution D, and stirred for 16 hours in the dark; the resulting product solution was added to 1500ml of ether In the m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com