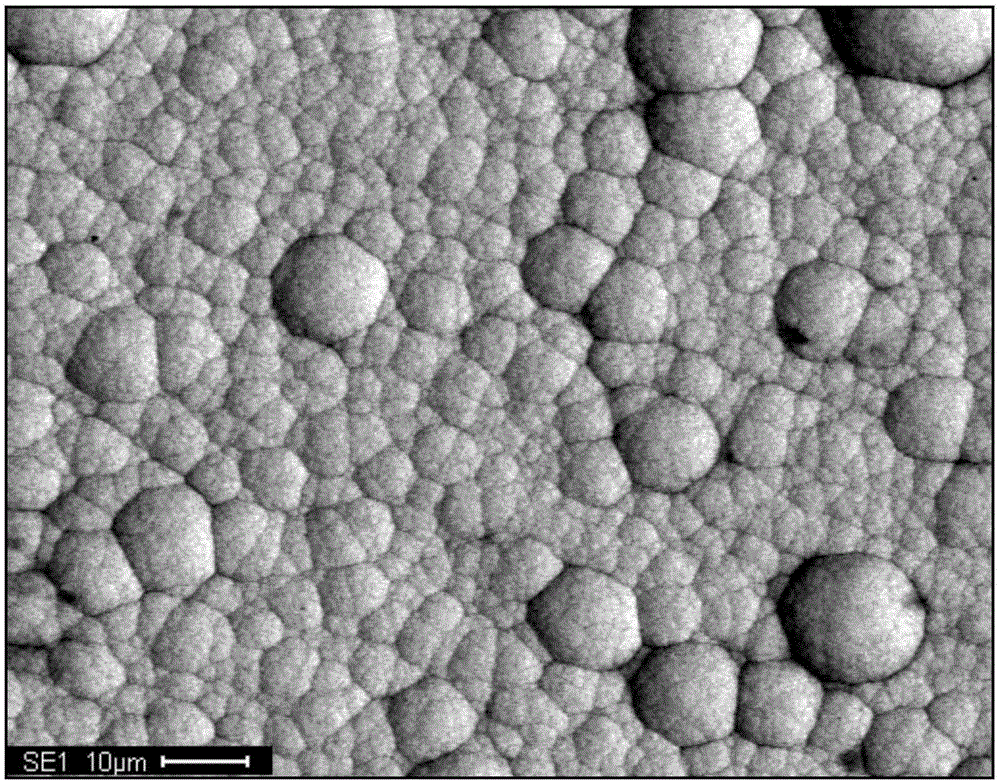
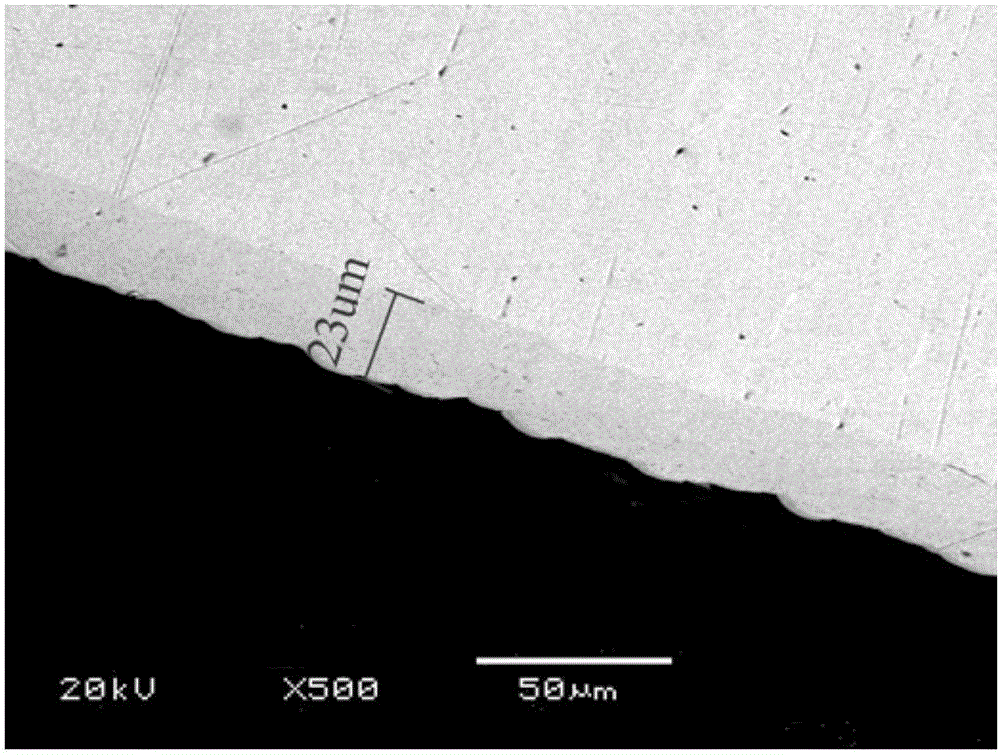
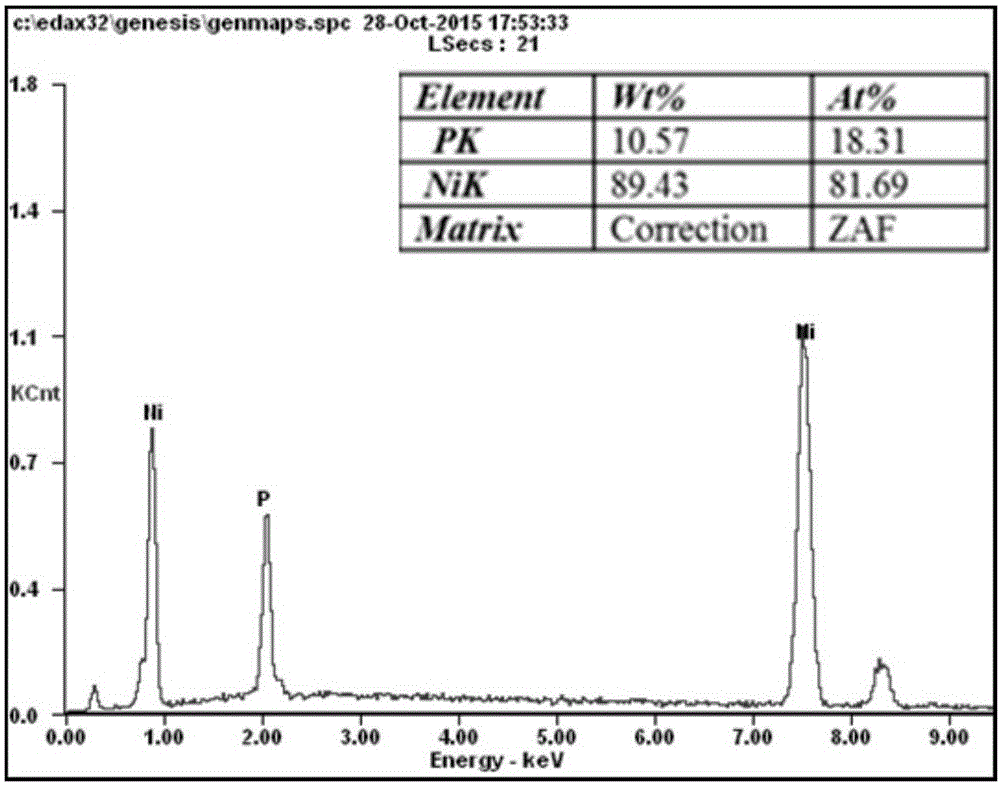
Solution for chemically plating nickel on surface of non-catalytic active material and plating process for solution
An electroless nickel plating, non-catalytic technology, applied in the field of electroless plating, can solve the problems of non-reusable plating solution, decreased plating deposition rate, reduced plating solution stability, etc., and achieves the effects of low cost, smooth surface and convenient configuration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Liquid A: 300g of nickel sulfate and 50g of ammonium fluoride per liter;
[0071] Solution B: Each liter contains 260g of sodium hypophosphite, 210g of citric acid monohydrate, 200g of ammonium fluoride, 0.02g of thiourea, and 0.15g of sodium lauryl sulfate. Take 10% of liquid A, 10% of liquid B and 80% of deionized water according to volume percentage, mix them evenly, adjust the pH to 7.7-8.0 with ammonia water, and form an electroless plating solution; pretreat the copper sheets that need to be nickel-plated, Then degrease with alkali washing solution for 3-5 minutes at 60°C-70°C, wash with water for the first time, pickle with acid at room temperature for about 30s, and wash with water for the second time; In the electroless plating solution at 88°C, the load of the plated parts is 0.6dm 2 / L, put the iron piece in contact with the copper piece for primer plating, take out the iron piece after the electroless plating occurs; take out the nickel-plated piece after 6...
Embodiment 2
[0074] Liquid A: 320g of nickel sulfate and 50g of ammonium fluoride per liter;
[0075] Liquid B: containing 250g of sodium hypophosphite, 200g of citric acid monohydrate, 200g of ammonium fluoride, 0.02g of thiourea, and 0.15g of sodium lauryl sulfate per liter;
[0076] Liquid D: 320g of nickel sulfate and 0.02g of thiourea per liter;
[0077] According to volume percentage, take 10% of liquid A, 10% of liquid B and 80% of deionized water, mix them evenly, adjust the pH to 7.7-8.0 with ammonia water, and form an electroless plating solution; put the processed parts to be plated into the heated In the electroless plating solution at 85°C, use an iron plate to lead the plating, and carry out nickel plating at a stable temperature of 85°C, with a load of 0.6dm 2 / L; after plating for 60 minutes, take out the nickel-plated parts, wash with water, dry or dry, filter the plating solution at the same time, and clean the plating tank; then add the above-mentioned plating solution ...
Embodiment 3
[0080] Liquid C: 480g of nickel sulfate and 100g of citric acid monohydrate per liter;
[0081] Carry out plating and replenishment by the method for embodiment 2, replenish twice and after carrying out electroless plating, after plating solution and plating tank are processed, the solution after plating continues to replenish for the third time, every liter of plating solution adds 20ml of solution C and 25ml of solution D, stir to mix the components of the plating solution evenly in the plating tank, adjust the pH to 7.7-8.0 with ammonia water, and obtain a new plating solution; heat the new plating solution to 85°C, and then add new parts to the plating solution Carry out electroless plating, lead plating with iron sheet, take out the plated parts that have been plated with nickel after plating for 60 minutes, wash with water, and blow dry. The plating solution can be recycled, and on the basis of the third replenishment, it can continue to add plating, add 20ml of C soluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com