Preparation method for Afatinib intermediate 6-nitryl-7-chlorol-4-quinazolinone
A technology of quinazolinone and afatinib, which is applied in the field of chemical synthesis, can solve the problems of high cost of formamidine acetate, difficulty in industrial production, environmental pollution, etc., and achieve the effects of low cost, reduced yield, and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
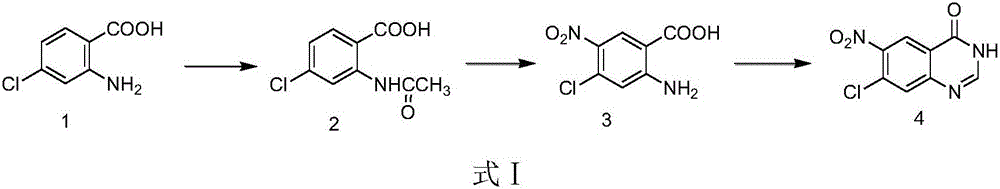
[0024] A preparation method of afatinib intermediate 6-nitro-7-chloro-4-quinazolinone, comprising the following steps:
[0025] Step (1): at room temperature, 2-amino-4-chlorobenzoic acid (100g, 0.583mol) was added to a 500ml round-bottom four-necked reaction flask, 300ml of acetic anhydride was added, vigorously stirred, refluxed for reaction, thin layer chromatography ( TLC) monitoring, the reaction was carried out for 5 hours; after the reaction, the reaction solution was poured into ice water, and a large amount of white solids were separated out, washed with water, and suction filtered to obtain 2-acetamido-4-chlorobenzoic acid (compound 2) 117g, yield 94.0 %. The product was used directly in the next reaction without purification.
[0026] The structural detection data of the 2-acetamido-4-chlorobenzoic acid prepared in this step are as follows:
[0027] m.p. is 208.2-208.9°C (literature value: 213-214°C);
[0028] 1 HNMR((CD3) 2 SO, 400MHz), δppm: 13.85(br,1H), 11....
Embodiment 2
[0036] Step (1) and step (3) are the same as in Example 1.
[0037] Step (2): under the condition of 20°C, add 30ml of concentrated sulfuric acid to the concentrated nitric acid (5.2g, 0.0536mol), and stir at room temperature for 1.5h to obtain a mixed acid system; under the condition of 8-10°C, add 2- Acetylamino-4-chlorobenzoic acid (10.4g, 0.0487mol) was reacted at room temperature and monitored by thin layer chromatography (TLC), after the reaction was completed, the reaction solution was poured into ice water, and a large amount of solid was precipitated; ethanol was recrystallized to obtain 0.53g The yellow solid was the product 2-amino-4-chloro-5-nitrobenzoic acid (compound 3), and the yield was 5.0%. The nuclear magnetic data and melting point data of the prepared 2-amino-4-chloro-5-nitrobenzoic acid are the same as those in Example 1.
Embodiment 3
[0039] Step (1) and step (2) are the same as in Example 1.
[0040] Step (3): under dry conditions, respectively, 2-amino-4-chloro-5-nitrobenzoic acid (3g, 13.85mmol), trimethyl orthoformate (6.4g, 60.67mmol), ammonium acetate (4.5g , 58.45mmol) was added to 50ml three-necked flask, then 20ml isopropanol was added, heated to reflux reaction, thin layer chromatography (TLC) monitoring, after the reaction was completed, the reaction solution was poured into ice water, washed with 100ml of water, suction filtered , and dried to obtain 1.9 g of 6-nitro-7-chloro-4-quinazolinone (compound 4) as a dark yellow solid with a yield of 61.0%. The nuclear magnetic data and melting point data of the prepared 6-nitro-7-chloro-4-quinazolinone are the same as those in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com