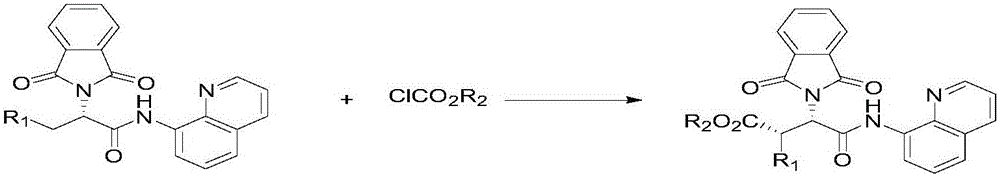Synthesis method of substituted aspartic acid
A technology of aspartic acid and synthesis method, applied in the direction of organic chemistry, etc., to achieve the effects of easy post-processing, simple operating conditions and high ee value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
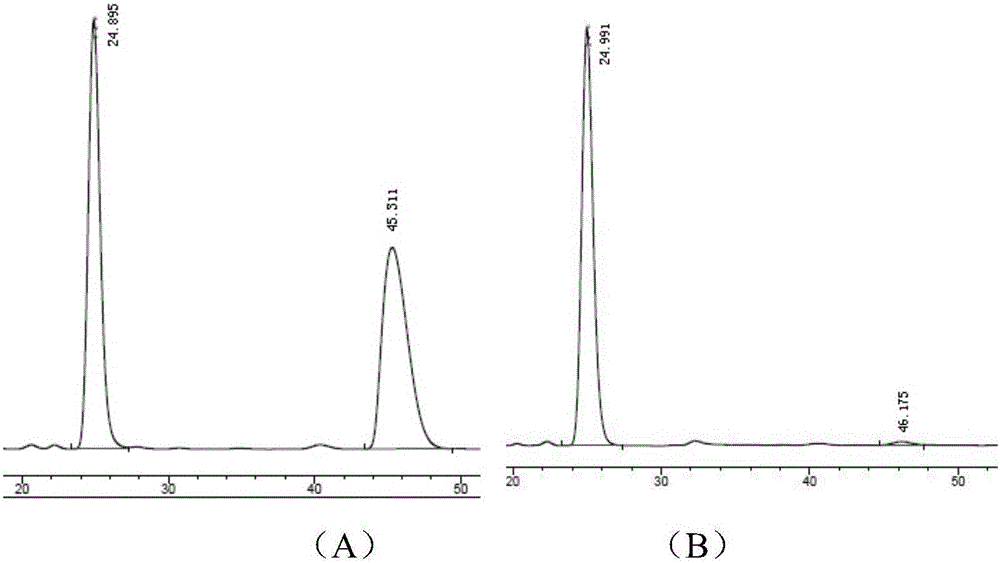
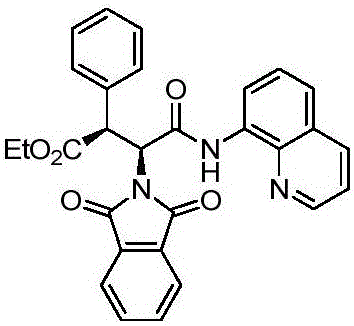
[0030] In the reactor, add 0.15 mmoles of L-phenylalanine whose nitrogen end is protected with phthaloyl group and carboxy end with 8-aminoquinoline, 0.45 mmoles of ethyl chloroformate 2a, 0.015 mmoles of palladium acetate catalyst , 0.3 mmol of silver carbonate, 0.15 mmol of elemental iodine and 2 milliliters of toluene, reacted in an air atmosphere at 120° C. for 16 hours and then finished the reaction for post-treatment. The product 3a with a structure such as formula 1 was obtained by silica gel column chromatography, and the yield was 76% with an ee of 97%.
[0031] The structure of product 3a is as follows:
[0032]
[0033] The structural characterization data are as follows:
[0034] 1 HNMR (400MHz, CDCl 3 )δ8.69(dd, J=4.1,1.6Hz,1H),8.28(dd,J=7.2,1.3Hz,1H),7.96(dd,J=8.3,1.6Hz,1H),7.83(dd,J =5.5,3.0Hz,2H),7.70(dd,J=5.4,3.1Hz,2H),7.54(dd,J=8.1,1.4Hz,1H),7.52–7.46(m,1H),7.42(d, J=7.2Hz, 2H), 7.25–7.12(m, 4H), 6.58(d, J=2.7Hz, 1H), 5.45(d, J=2.7Hz, 1H). 13 CNMR (1...
Embodiment 2~16
[0045] The operation steps are the same as in Example 1, the difference is that different aspartic acid derivatives can be obtained by changing the substituent on the aromatic ring of the raw material (see Table 3).
[0046] The structural formula of the raw material is as follows:
[0047]
[0048] The experimental results of the synthetic aspartic acid derivatives of table 3 embodiment 2~16
[0049]
Embodiment 17
[0051] In the reactor, add 0.15 mmoles of substituted L-phenylalanine 1q, 0.45 mmoles of ethyl chloroformate 2a whose nitrogen end is protected with phthaloyl group and carboxyl end with 8-aminoquinoline , 0.015 millimole palladium acetate catalyst, 0.3 millimole silver carbonate, 0.15 millimole elemental iodine and 2 milliliters of toluene, after reacting in 120 ℃ of air atmospheres for 16 hours, finish reaction and carry out aftertreatment, obtain as structure formula 2 by silica gel column chromatography The yield of product 3q was 73%. 1 HNMR (400MHz, CDCl 3 ):δ10.15(s,1H),8.68(dd,J=6.7,2.2Hz,1H),8.51(d,J=3.1Hz,1H),8.05(d,J=8.2Hz,1H),7.75 (dd,J=5.4,3.1Hz,2H),7.65(dd,J=5.4,3.1Hz,2H),7.50–7.43(m,2H),7.31(dd,J=8.2,4.2Hz,1H), 6.87(d, J=2.0Hz, 1H), 6.79(dd, J=8.4, 2.1Hz, 1H), 6.64(d, J=8.3Hz, 1H), 5.94(d, J=11.5Hz, 1H), 4.92(d, J=11.5Hz, 1H), 4.28(dq, J=10.7, 7.1Hz, 1H), 4.21–4.02(m, 5H), 1.24(t, J=7.1Hz, 3H); 13 CNMR (101MHz, CDCl 3 )δ 172.1, 167.5, 166.0, 148.4, 143.5, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com