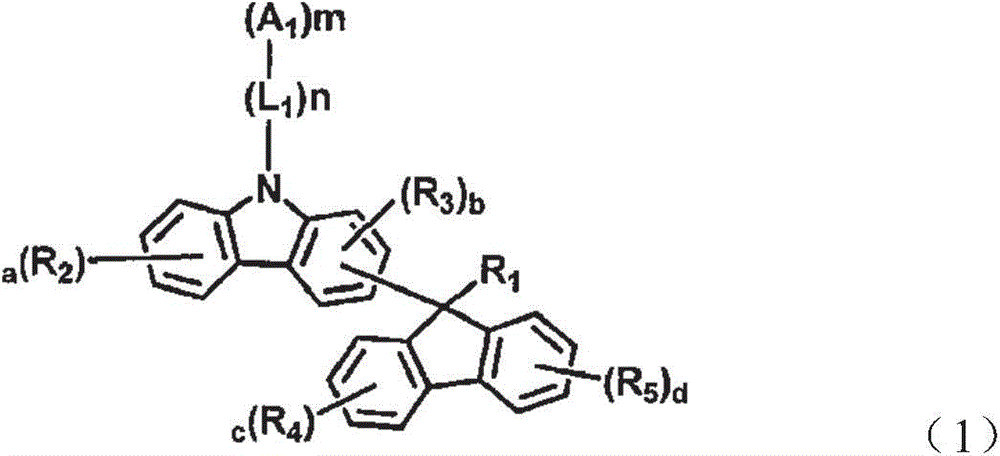
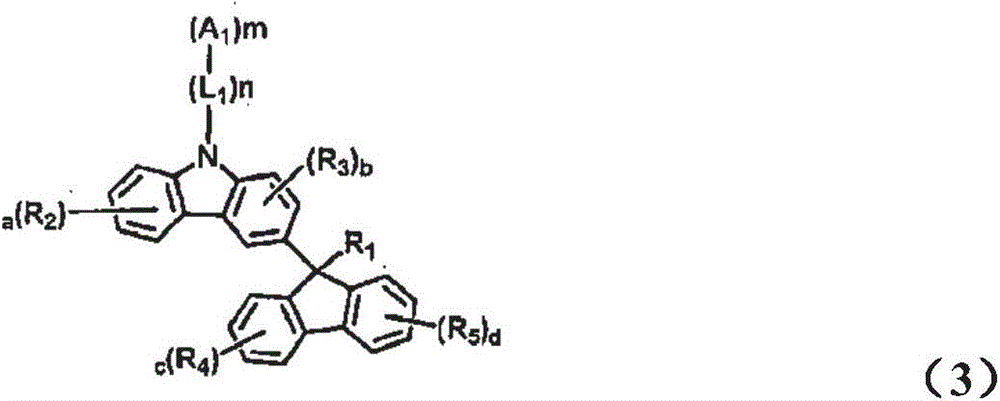
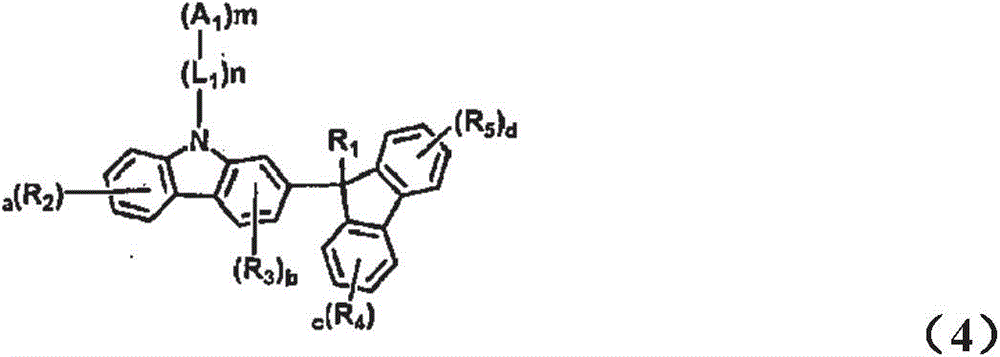
Novel organic electroluminescent compounds and organic electroluminescent device comprising the same
An electroluminescent device and compound technology, which can be used in organic chemistry, compounds of Group 4/14 elements of the periodic table, luminescent materials, etc. Satisfaction and other issues, to achieve the effect of reducing power consumption and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0105] Example 1: Preparation of compound H-34
[0106]
[0107] Preparation of Compound 1-2
[0108] 2-Bromobiphenyl (50.0 g, 214.0 mmol) and tetrahydrofuran (THF) (1.0 L) were cooled to -78 °C in a 3 L round bottom flask (RBF) and 2.5 M n-butyllithium (103.0 mL, 257.0 mmol). After 2 hours, (4-bromophenyl)(phenyl)methanone (56.0 g, 214.0 mmol) was added to the flask. After 17 hours, with dichloromethane (MC) and H 2 O extracted the mixture, and subjected the MC layer to MgSO 4 dry. The MC layer was concentrated to obtain compound 1-1.
[0109] Compound 1-1, hydrochloric acid (100.0 mL) and acetic acid (1.0 L) were stirred under reflux in a 3LRBF. After 14 hours, the resulting solid was filtered, and the filtered solid was dissolved in chloroform (CHCl 3 ), and applied to column chromatography to obtain compound 1-2 (35.0 g, 42%).
[0110] Preparation of Compounds 1-3
[0111] Compound 1-2 (35.0 g, 89.0 mmol), bis(pinacolate) diborane (27.0 g, 106.0 mmol), bi...
example 2
[0123] Example 2: Preparation of Compound H-57
[0124]
[0125] Preparation of compound 2-1
[0126] Compound 1-3 (35.0 g, 78.0 mmol), 2,5-dibromonitrobenzene (26.2 g, 93.0 mmol), Pd(PPh 3 ) 4 (3.6g, 3.1mmol), Na 2 CO 3 (20.6g, 195.0mmol), toluene (400.0mL), EtOH (50.0mL) and H 2 O (100.0 mL) overnight. with ethyl acetate (EA) and H 2 O treated the reaction mixture with MgSO 4 Water was removed, and the residue was distilled under reduced pressure. The crude product was applied to column chromatography with MC:hexane (Hx) to obtain compound 2-1 (30.0 g, 75%) as a solid.
[0127] Preparation of compound 2-2
[0128] Compound 2-1 (30.0 g, 57.8 mmol), P(OEt) was stirred in 1 LRBF at 150 °C 3 (200.0 mL) and 1,2-DCB (200.0 mL) for 2 hours. The reaction mixture was distilled to obtain a solid. The crude product was applied to column chromatography with MC:Hx to obtain compound 2-2 (19.0 g, 68%) as a white solid.
[0129] Preparation of Compound 2-3
[0130]...
example 3
[0134] Example 3: Preparation of Compound H-90
[0135]
[0136] Preparation of compound 3-1
[0137] 9-Fluorenone (20.0 g, 111.0 mmol) was dissolved in THF (554.0 mL) in a flask and phenylmagnesium bromide (36.9 mL) was slowly added thereto at 0°C. The mixture was stirred at room temperature for 24 hours. After the reaction was complete, the organic layer was extracted with EA and 4 Remove remaining moisture and dry. The layers were separated by column chromatography to obtain compound 3-1 (20.0 g, 70%).
[0138] Preparation of compound 3-2
[0139] 2-Bromo-9H-carbazole (20.0 g, 81.2 mmol), phenylboronic acid (11.9 g, 97.5 mmol), Pd(PPh 3 ) 4 (4.7g, 4.06mmol), 2MK 2 CO 3 (121.0 mL), toluene (250.0 mL), and EtOH (121.0 mL) for 5 hours. After the reaction was complete, the organic layer was extracted with EA and 4 Remove remaining moisture and dry. The layers were separated by column chromatography to obtain compound 3-2 (17.0 g, 86%).
[0140] Preparatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com