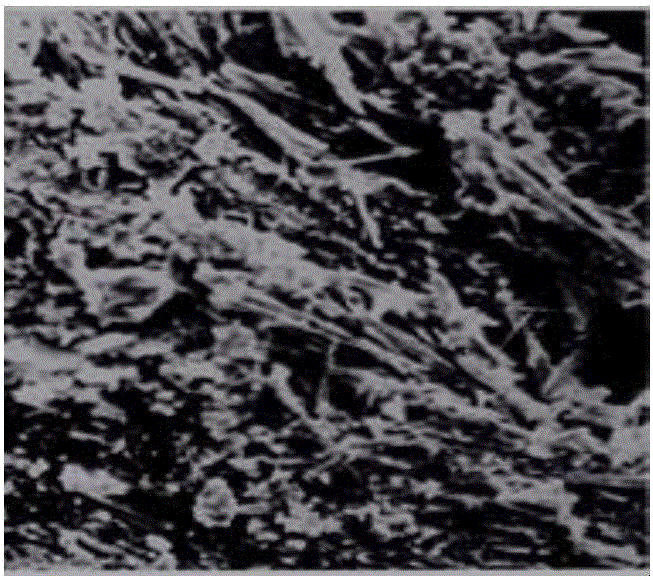
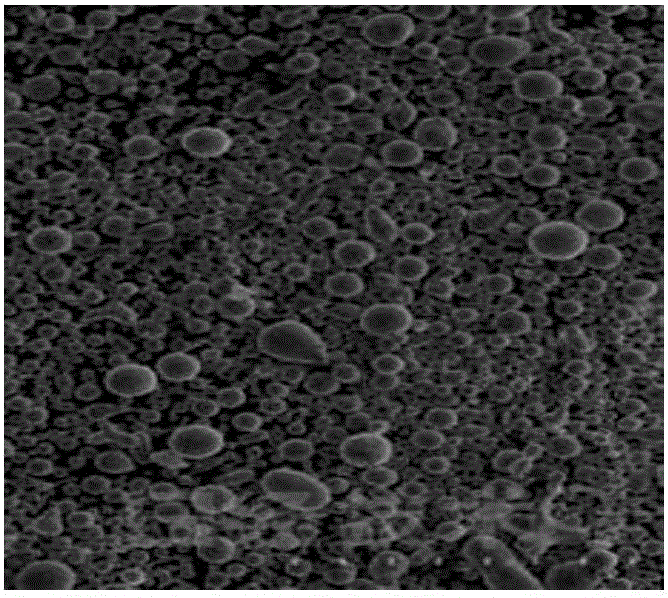
Lysozyme hydrochloride vaginal tablets, and preparation method and application thereof
A technology of lysozyme hydrochloride and vaginal tablets, which is applied in the field of medicine, can solve the problems of high potency and undescribed, and achieve the effects of decomposing pus, improving mucopolysaccharide metabolism, and enhancing curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] A preparation method of lysozyme hydrochloride vaginal tablet, comprising the steps of:
[0032] (1) Grinding the lactose and passing through a 80-100 mesh sieve;
[0033] (2) Weigh hydrochloric acid lysozyme according to the proportion, add purified water to dissolve, adjust pH to 4.0-6.0 with 2-10% (W / W) hydrochloric acid, filter and sterilize with 0.1-0.22 μm filter membrane, and lyse the obtained hydrochloric acid The enzyme feed solution is transported to the sterilized tank and kept at room temperature for standby;
[0034] (3) Sterilize lactose and magnesium stearate separately at a temperature of 40-60°C, control the sterilization pressure of lactose at 20-30Kpa, and the time of 6-7h; control the sterilization pressure of magnesium stearate at 35-50Kpa , the time is 3~4h, standby;
[0035] (4) Weigh mannitol according to the proportion, raise the temperature to 131-136° C., and keep the temperature for 20-30 minutes for sterilization; transport it to a sterili...
experiment example 1
[0078] Experimental Example 1, Lactose and Magnesium Stearate Sterilization Experiment
[0079] See Table 1 for the comparison of different ethylene oxide sterilization pressures and times on the sterilization effects of lactose and magnesium stearate.
[0080] Table 1 Comparison of sterilization effects of lactose and magnesium stearate
[0081]
[0082] It can be analyzed from Table 1 that:
[0083] 1. Under the conditions of ethylene oxide sterilization pressure of 10-20Kpa and time of 3-6 hours, the sterility test of lactose and magnesium stearate did not meet the requirements.
[0084] 2. Under the conditions of ethylene oxide sterilization pressure of 20-30Kpa and time of 3-4 hours, lactose and magnesium stearate sterility test did not meet the requirements.
[0085] 3. Under the conditions of ethylene oxide sterilization pressure of 20-30Kpa and time of 6 hours, the sterility test of lactose meets the regulations. But the sterility test of magnesium stearate did n...
experiment example 2
[0088] Experimental example 2, mannitol sterilization experiment
[0089] The comparison of different high-temperature sterilization temperatures and times on the high-temperature sterilization effect of mannitol is shown in Table 2:
[0090] Table 2 Comparison of mannitol high temperature sterilization effect
[0091]
[0092] It can be analyzed from Table 2 that:
[0093] 1. Under the conditions of high-temperature sterilization at 121-126°C and 15-30 minutes, the sterility test does not meet the requirements;
[0094] 2. The high temperature sterilization temperature is 131℃, the time is 15min, and the sterility test does not meet the requirements;
[0095] 3. The high temperature sterilization temperature is 131~136℃, the time is 20~30min, and the sterility inspection meets the regulations;
[0096] Test results: Mannitol was sterilized at high temperature by controlling the temperature at 131-136°C and the time at 20-30 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com