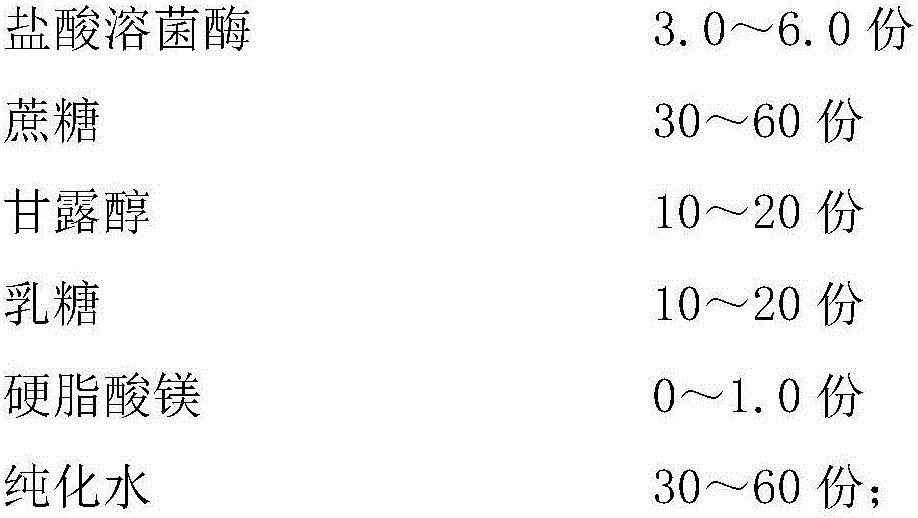Composite hydrochloric acid lysozyme vagina tablet and preparation method thereof
A technology of lysozyme hydrochloride and compound hydrochloric acid, which is applied in the field of medicine, can solve the problems of undescribed and high potency, and achieve the effects of enhancing local defense, improving metabolism, and improving local circulation disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
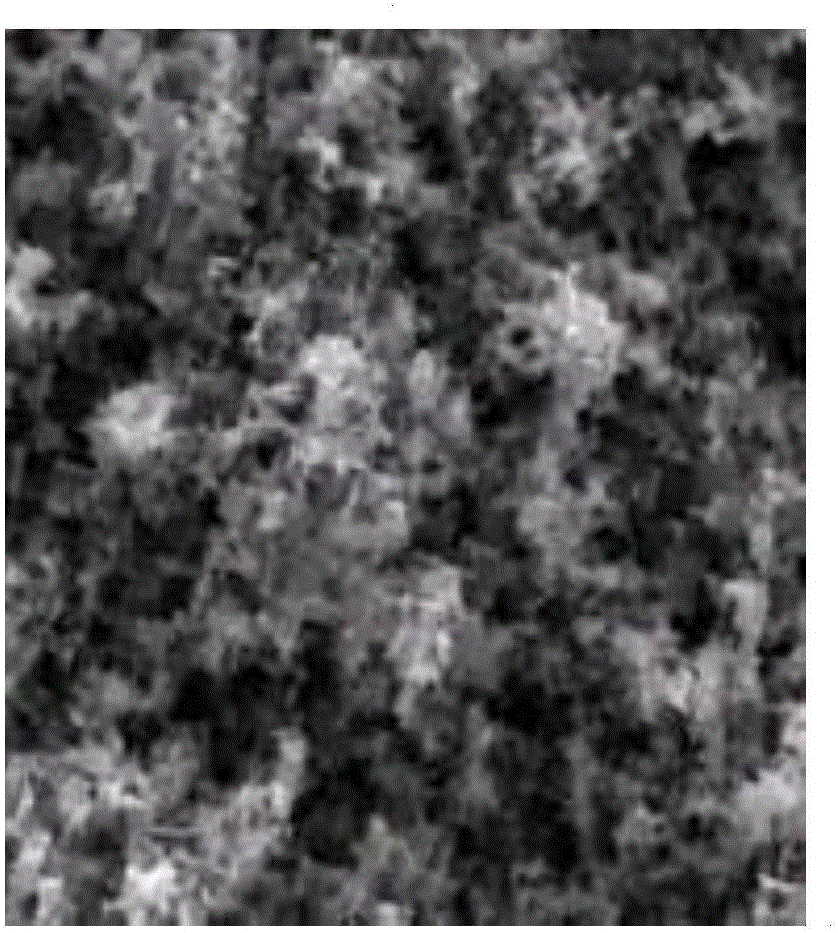
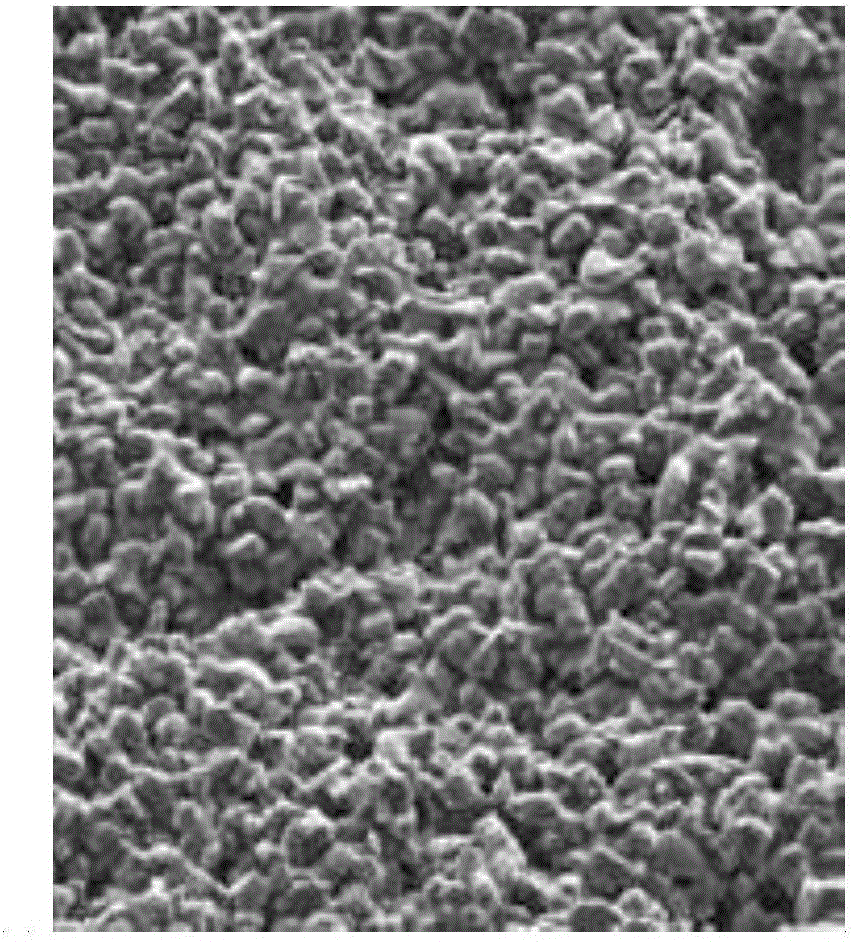
Image
Examples
preparation example Construction
[0034] A preparation method of compound lysozyme hydrochloride vaginal tablet, comprising the steps of:
[0035] (1) Pulverize sucrose and pass through a 100-120 mesh sieve; pulverize lactose and pass through a 80-100 mesh sieve; sterilize sucrose, lactose and magnesium stearate at a temperature of 40-60°C respectively: sucrose, The sterilization pressure of lactose is controlled at 20-30Kpa, and the time is 6-7h; the sterilization pressure of magnesium stearate is controlled at 35-50Kpa, and the time is 3-4h, and it is used for standby;
[0036] (2) Weigh hydrochloric acid lysozyme according to the proportion, add purified water to dissolve, adjust the pH to 4.0-6.0 with 2-10% (W / W) hydrochloric acid, filter and sterilize the obtained lysozyme with a 0.1-0.22 μm filter membrane The feed liquid is transported to the sterilized tank and kept at room temperature for standby;
[0037] (3) Weigh and mix mannitol and purified water according to the proportion, continue to heat up ...
experiment example 1
[0087] Experimental example 1, sucrose and auxiliary material sterilization experiment
[0088] See Table 1 for the comparison of different ethylene oxide sterilization pressures and times on the sterilization effects of sucrose and magnesium stearate.
[0089] Table 1 Comparison of sterilizing effects of sucrose and magnesium stearate
[0090]
[0091] It can be analyzed from Table 1 that:
[0092] 1. Under the conditions of ethylene oxide sterilization pressure of 10-20Kpa and time of 3-6 hours, the sterility test of sucrose, lactose and magnesium stearate did not meet the requirements;
[0093] 2. Under the conditions of ethylene oxide sterilization pressure of 20-30Kpa and time of 3-4 hours, the sterility test of sucrose, lactose and magnesium stearate did not meet the requirements;
[0094] 3. Under the conditions of ethylene oxide sterilization pressure 20-30Kpa, time 6h, sucrose, lactose sterility inspection, meet the regulations. But the sterility test of magnesium...
experiment example 2
[0097] Experimental example 2, mannitol sterilization experiment
[0098] The comparison of different high-temperature sterilization temperatures and times on the high-temperature sterilization effect of mannitol is shown in Table 2:
[0099] Table 2 Comparison of mannitol high temperature sterilization effect
[0100]
[0101] It can be analyzed from Table 2 that:
[0102] 1. Under the conditions of high-temperature sterilization at 121-126°C and 15-30 minutes, the sterility test does not meet the requirements;
[0103] 2. The high temperature sterilization temperature is 131℃, the time is 15min, and the sterility test does not meet the requirements;
[0104] 3. The high temperature sterilization temperature is 131~136℃, the time is 20~30min, and the sterility inspection meets the regulations;
[0105] Test results: Mannitol was sterilized at high temperature by controlling the temperature at 131-136°C and the time at 20-30 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com