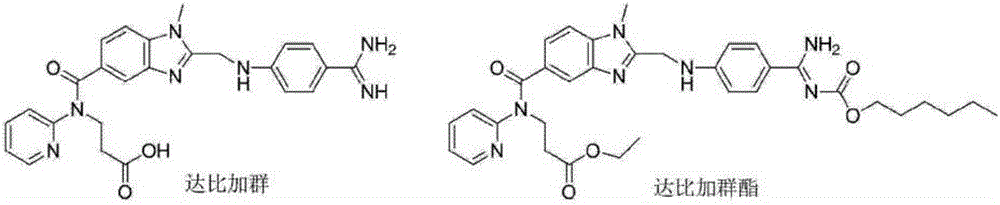
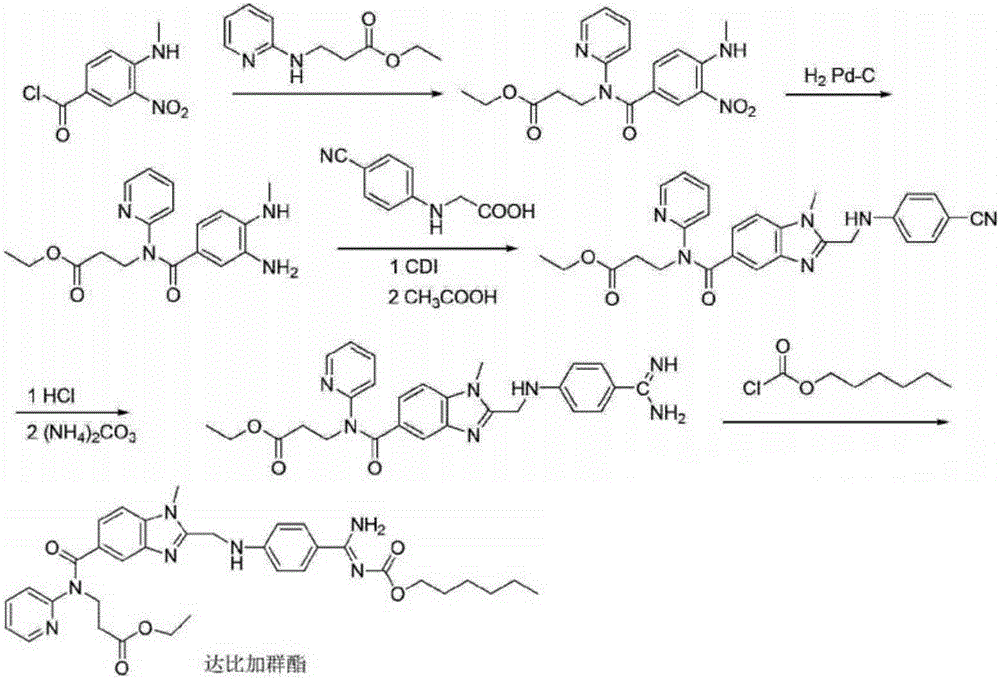
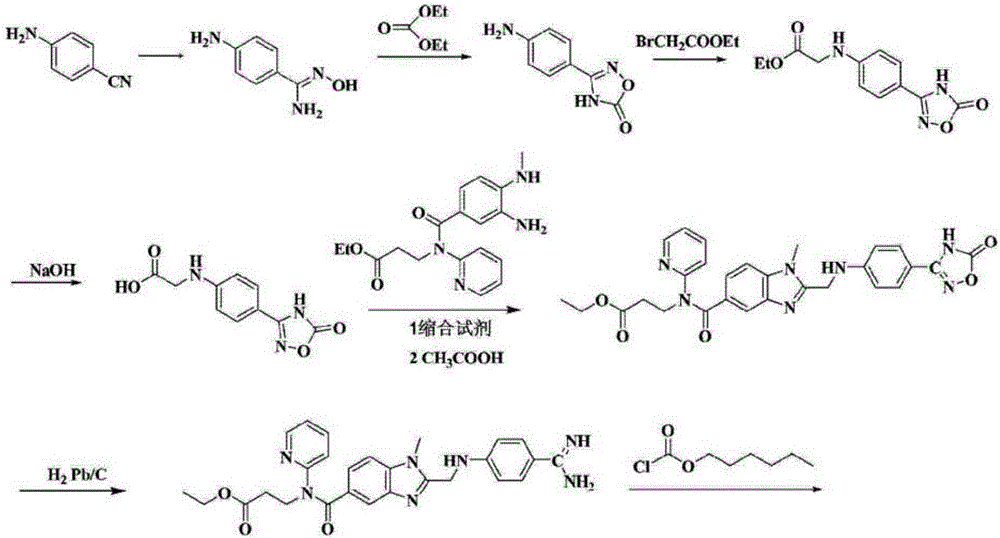
Method for synthesizing dabigatran etexilate intermediate
A synthesis method and dropwise addition technology, applied in the direction of organic chemistry, etc., can solve the problems of high impurities in intermediates, difficult separation and purification, low yield of synthesis route 1, etc., and achieve the effect of high atomic economic benefit.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] In a preferred embodiment, the synthesis method comprises the following steps:
[0074] (1) Using 3-nitro-4-methylaminobenzoic acid (II) as the starting material, dissolve it in a solvent, and treat it with thionyl chloride to obtain acid chloride; then esterify with methanol in the presence of a base Reaction generates 3-nitro-4-methylaminobenzoic acid methyl ester (Ⅲ);
[0075] Wherein, the solvent is selected from any one of toluene, benzene or chlorobenzene, and the base is selected from any one of triethylamine, diisopropylethylamine or N, N-dimethylaminopyridine;
[0076] (2) Methyl 3-nitro-4-methylaminobenzoate (Ⅲ) is dissolved in a solvent, and palladium carbon or Raney nickel is used as a catalyst, and catalytic hydrogenation is carried out under a hydrogen pressure of 1-10 times the atmospheric pressure Reduction reaction generates 3-amino-4-methylaminobenzoic acid methyl ester (Ⅳ);
[0077] Wherein, the solvent is selected from any one of tetrahydrofuran, e...
Embodiment 1
[0107] Synthesis of Methyl 3-Nitro-4-Methylaminobenzoate (Ⅲ)
[0108] Under nitrogen protection, put 3-nitro-4-methylaminobenzoic acid (100g, 0.51mol), N,N-dimethylformamide (catalytic amount: 2 drops), toluene (500mL ). Turn on the heating and raise the temperature to 90°C. When the system is at 60°C, start to add thionyl chloride (91g, 0.76mol) dropwise, and the dropwise addition is completed in about half an hour. After dropping, keep warm at 90°C for reaction, and continue stirring for 1 hour after the system is clarified. Then toluene and thionyl chloride were concentrated. After the concentration, methanol (300 mL) was added to the concentrate. The temperature was raised to 60-65° C., and triethylamine (154.8 g, 1.53 mol) was added dropwise. After dropping the incubation reaction, HPLC followed the reaction for about 1 hour. The reaction solution was concentrated to dryness, and the concentrate was dissolved in dichloromethane (500 mL). It was washed successively w...
Embodiment 2
[0113] Synthesis of Methyl 3-Amino-4-Methylaminobenzoate (Ⅳ)
[0114] Methyl 3-nitro-4-methylaminobenzoate (95 g, 0.45 mol) and tetrahydrofuran (950 mL) were put into a hydrogenation reactor. The temperature was raised to 60° C., and after the system was clarified, 10% palladium carbon (9.5 g) was added. First replace the air in the reactor with nitrogen, and then replace the nitrogen with hydrogen. The hydrogenation was started, and the reaction progress was tracked by TLC, and the reaction was completed in 2 hours. The temperature of the system was lowered to below 40°C. Palladium carbon was filtered off, and the filter cake was washed twice with a small amount of tetrahydrofuran (50 mL). The filtrates were combined and concentrated to dryness under vacuum at 50° C. to obtain 76 g of methyl 3-amino-4-methylaminobenzoate as an off-white solid, with a yield of 93.3%.
[0115] MP: 148.1-153.6°C
[0116] LC-MS: Theoretical C 9 h 12 N 2 o 2 (M+1)181, the actual value is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com