N-indazole substituted thiourea derivatives and preparation method and application thereof
A technology of derivatives and thioureas, applied in the field of chemical medicine, to achieve the effect of high antiviral activity and good antioxidant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0053] The present invention will be further described in detail below in conjunction with specific embodiments, which are explanations of the present invention rather than limitations.
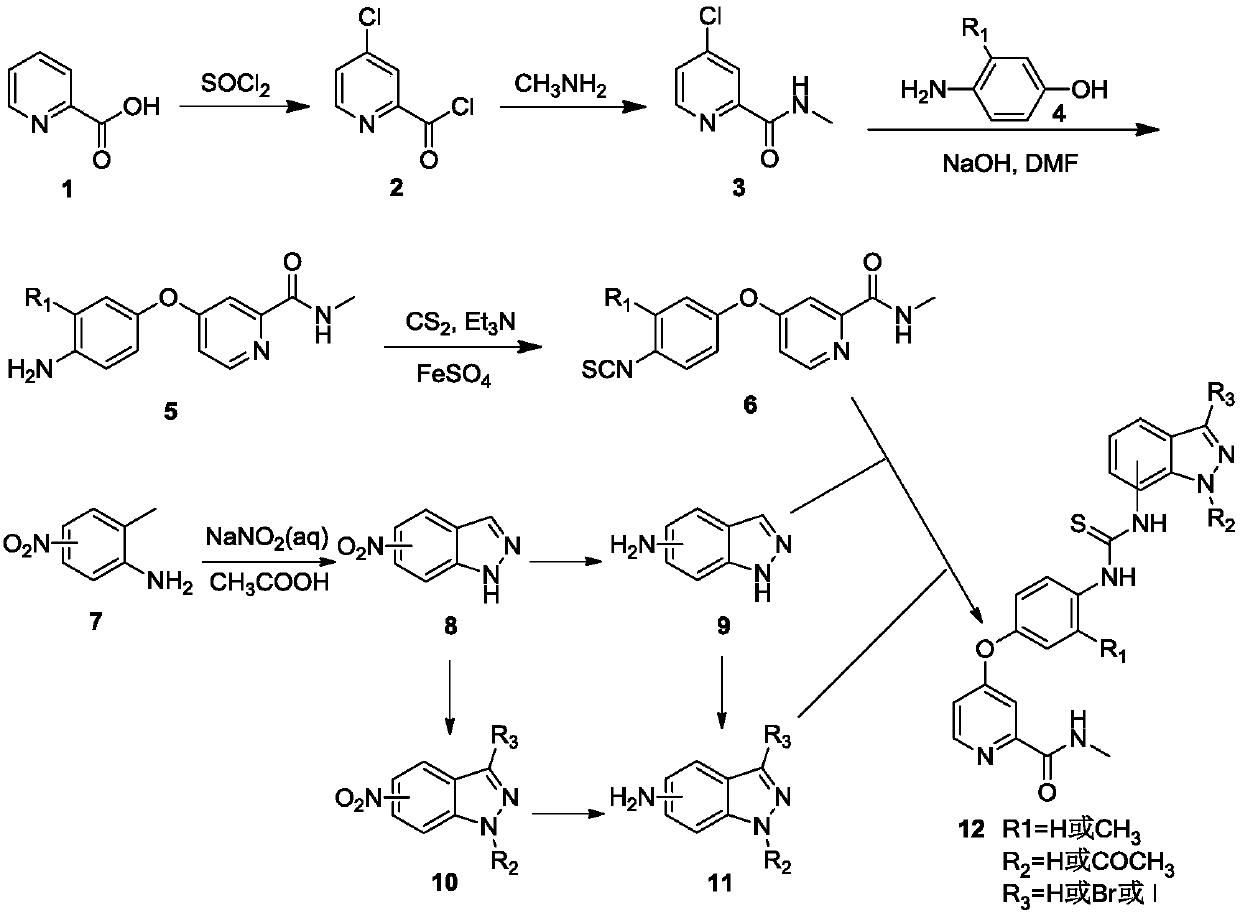
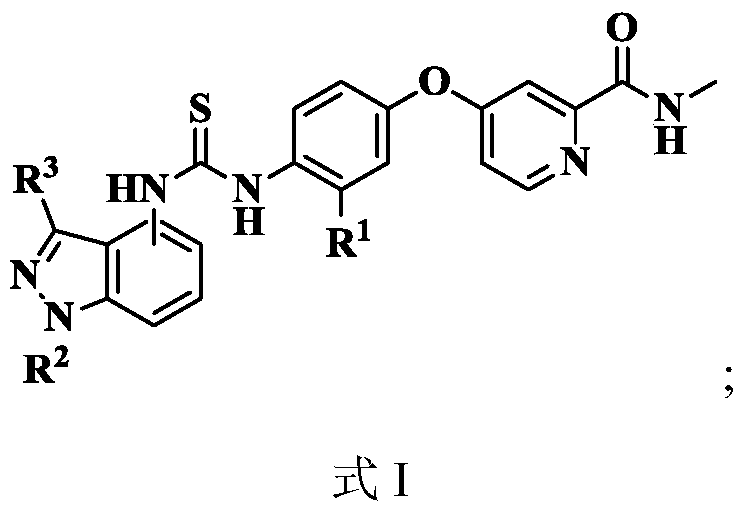
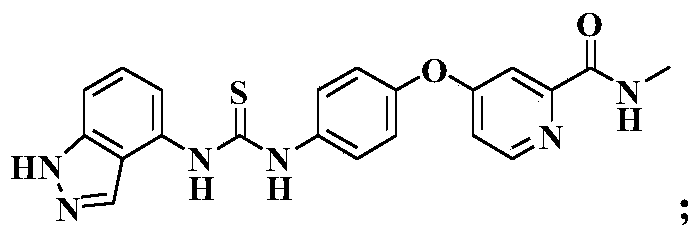
[0054] see figure 1 , the present invention takes 2-pyridinecarboxylic acid 1 as raw material, reacts in thionyl chloride to generate intermediate 2 (4-chloropyridine-2-formyl chloride); intermediate 2 reacts with methylamine solution at low temperature, and undergoes hydrogen oxidation Alkalinization of sodium solution generates intermediate 3 (N-methyl-4-chloropyridine-2-carboxamide); intermediate 3 and p-aminophenol 4 react to generate intermediate 5 [4-(4 -aminophenoxy)-N-methyl-2-pyridinecarboxamide]; intermediate 5 reacts to generate N-methyl-4-(4-isosulfur in the presence of carbon disulfide, triethylamine and ferrous sulfate Cyanate (phenoxy)pyridine-2-carboxamide 6. In addition, 2-methylaniline 7 substituted with nitro in different positions is used as starting material, and glacia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com