A method for improving the bioavailability of artemisinin drugs
A technology for artemisinin and drugs, which is applied in the field of improving the bioavailability of artemisinin drugs, and can solve problems such as low bioavailability, affecting drug efficacy, and unstable chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Preparation of DHA bulk drug (dihydroartemisinin bulk drug) powder processed by SCF:
[0090] Supercritical fluid equipment of Tianjin Crystec Pharmaceutical Technology Co., Ltd. (composition: 200mL particle forming container, 50g / min-capacity CO 2 Pump). 2% DHA-ethanol solution (w / v), with CO 2 Pump into appropriate nozzles (coaxial nozzles), maintain a pressure of 85 bar (back pressure regulator control) and an operating temperature of 40°C, and finally collect the final powder in the forming chamber.
[0091] Characterization of SCF-treated DHA API powders:
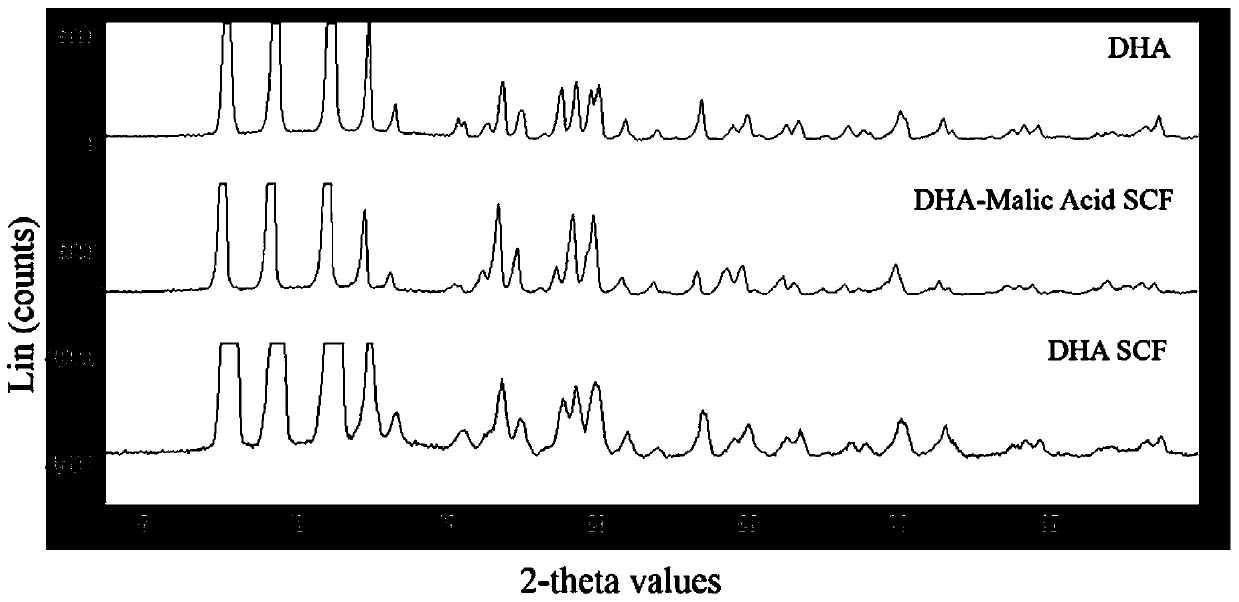
[0092] (1) Powder X-ray Diffraction (PXRD) characterization: Simultaneously compare and investigate the DHA bulk drug, experimental instrument parameters: 40kV, 40mA; scanning speed 4θ / min. see results figure 1 . Both the DHA raw drug and the DHA raw drug treated with SCF have strong similar diffraction peaks at the same position, but the peak number and peak intensity are changed, and the crystallinity is ...
Embodiment 2
[0095] The preparation of the DHA+malic acid powder that SCF handles: the supercritical fluid equipment (composition: 200mL granule forming container, the CO of 50g / min-capacity 2 Pump). 12% (w / v) DHA and 8% (w / v) malic acid in dichloromethane-tetrahydrofuran (volume ratio 3:1) solution, with CO 2 Pump into appropriate nozzles, maintain a pressure of 85 bar and an operating temperature of 40°C, and finally collect the final powder in the forming chamber.
[0096] (1) Powder X-ray Diffraction (PXRD) characterization: Experimental instrument parameters: 40kV, 40mA; scanning speed 4θ / min. see results figure 1 , compared with the DHA bulk drug, there are stronger similar diffraction peaks at the same position, but the number of peaks and the intensity of the peaks change to some extent, and the crystallinity is stronger.
[0097] (2) Scanning electron microscope (SEM) characterization: see the results Figure 4 , all form good needle crystals, the average length of the "needle...
Embodiment 3
[0113] The preparation of the DHA+gentisic acid co-crystallization powder that SCF handles: the supercritical fluid equipment (composition: 200mL granule forming container, the CO of 50g / min-capacity 2 Pump). 5% DHA + 2% gentisic acid in dichloromethane, with CO 2 Pump into appropriate nozzles, maintain a pressure of 85 bar and an operating temperature of 36°C, and finally collect the final powder in the forming chamber. Scanning electron microscope (SEM) characterization results are shown in Figure 6 . The average crystal length is 71 μm, and the geometric equivalent diameter (particle size) is 10 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com