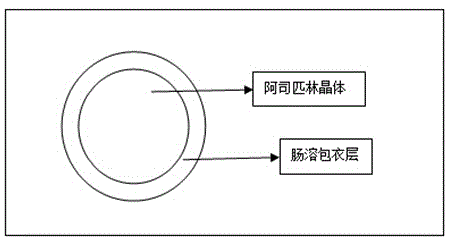
Preparation method of aspirin enteric microencapsulated capsule
A technology for aspirin and enteric-coated microparticles, which is applied in the field of preparation of aspirin enteric-coated microparticle capsules, can solve the problems of complex process, difficult to control aspirin degradation, etc., and achieves a technology with few process steps, which is beneficial for patients to swallow and easy to control process parameters. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Aspirin enteric-coated microparticle capsules (according to 10000 capsules)
[0038]
[0039] (1) Material preparation: Weigh 20-100 mesh aspirin crystals, Eudragit® L30D-55, triethyl citrate and talcum powder according to the prescription, and set aside;
[0040] (2) Preparation of coating solution: Add triethyl citrate and talcum powder into purified water under stirring, mix homogeneously for 5-10 minutes, then add Eudragit? L30D-55, continue stirring for 30-60 minutes, 40 mesh Filter to obtain the coating liquid;
[0041] (3) Coating; put the aspirin crystals weighed in step (1) into the fluidized bed granulation coating machine, adjust the air intake frequency to 10-30HZ, the material temperature to 25-35°C, and the atomization pressure to 0.15-0.2Mpa, Add the coating solution in step (2) for coating, and the weight of the coating will increase by 30-70%, to obtain aspirin crystal enteric-coated particles;
[0042] (4) Capsule filling: Aspirin cryst...
Embodiment 2
[0043] Example 2: Aspirin enteric-coated microparticle capsules (according to 10000 capsules)
[0044]
[0045](1) Material preparation: Weigh 20-100 mesh aspirin crystals, Kollicoat® MAE30DP, triethyl citrate, glyceryl monostearate and Tween 80 according to the prescription, and set aside;
[0046] (2) Preparation of coating solution: Add Tween 80, glyceryl monostearate and triethyl citrate into 250g of hot purified water, mix well for 10 minutes, add the remaining purified water to glyceryl monostearate Stir in the emulsion to room temperature, then add to Kollicoat® MAE30DP, stir continuously for 30-60 minutes, filter through 40 mesh to obtain the coating solution;
[0047] (3) Coating; put the aspirin crystals obtained in step (1) into the fluidized bed granulation coating machine, adjust the air intake frequency to 10-30HZ, the material temperature to 25-35°C, the atomization pressure to 0.15-0.2Mpa, add The coating solution in step (2) is coated, and the weight of th...
Embodiment 3
[0049] Example 3: Aspirin enteric-coated microparticle capsules (according to 10000 capsules)
[0050] (1) Material preparation: Weigh 20-100 mesh aspirin crystals, Kollicoat® MAE100P, polyethylene glycol and talcum powder according to the prescription, and set aside;
[0051] (2) Preparation of coating solution: Slowly add Kollicoat®MAE100P to 600g of purified water under stirring, slowly add sodium hydroxide solution dropwise after stirring for 5-10 minutes, continue stirring for 30 minutes, add polyethylene glycol and talc powder into purified water , mix evenly for 5-10 minutes, then add Kollicoat® MAE100P water dispersion, stir continuously for 30-60 minutes, filter at 40 mesh to obtain the coating solution;
[0052] (3) Coating; put the aspirin crystals obtained in step (1) into the fluidized bed granulation coating machine, adjust the air intake frequency to 10-30HZ, the material temperature to 25-35°C, the atomization pressure to 0.15-0.2Mpa, add The coating solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com