A kind of iron oxide catalyst and its preparation and application
A technology of catalyst and iron oxide, which is applied in the direction of physical/chemical process catalyst, metal/metal oxide/metal hydroxide catalyst, organic compound preparation, etc. It can solve the problems of poor stability and achieve high conversion rate and good propane The effect of acid selectivity and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
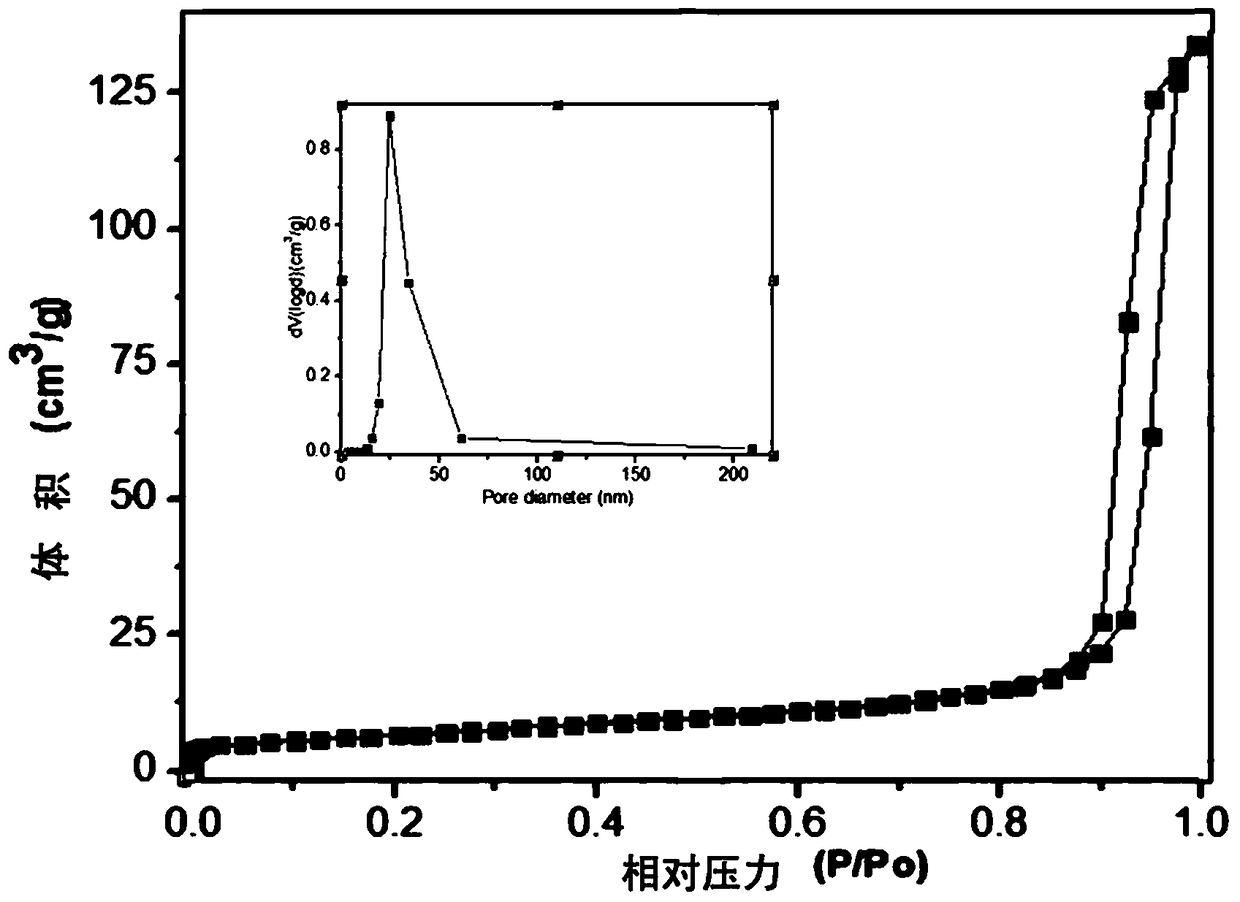
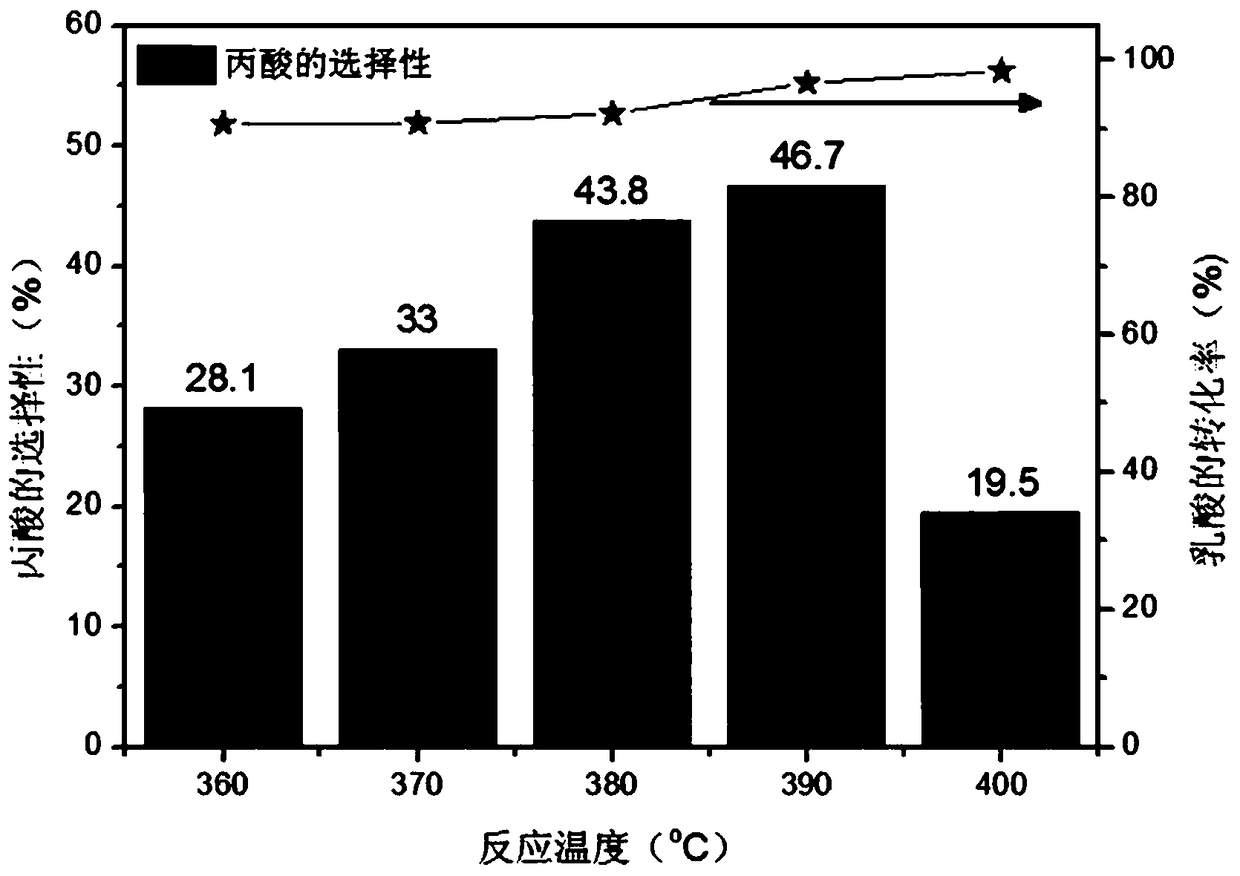
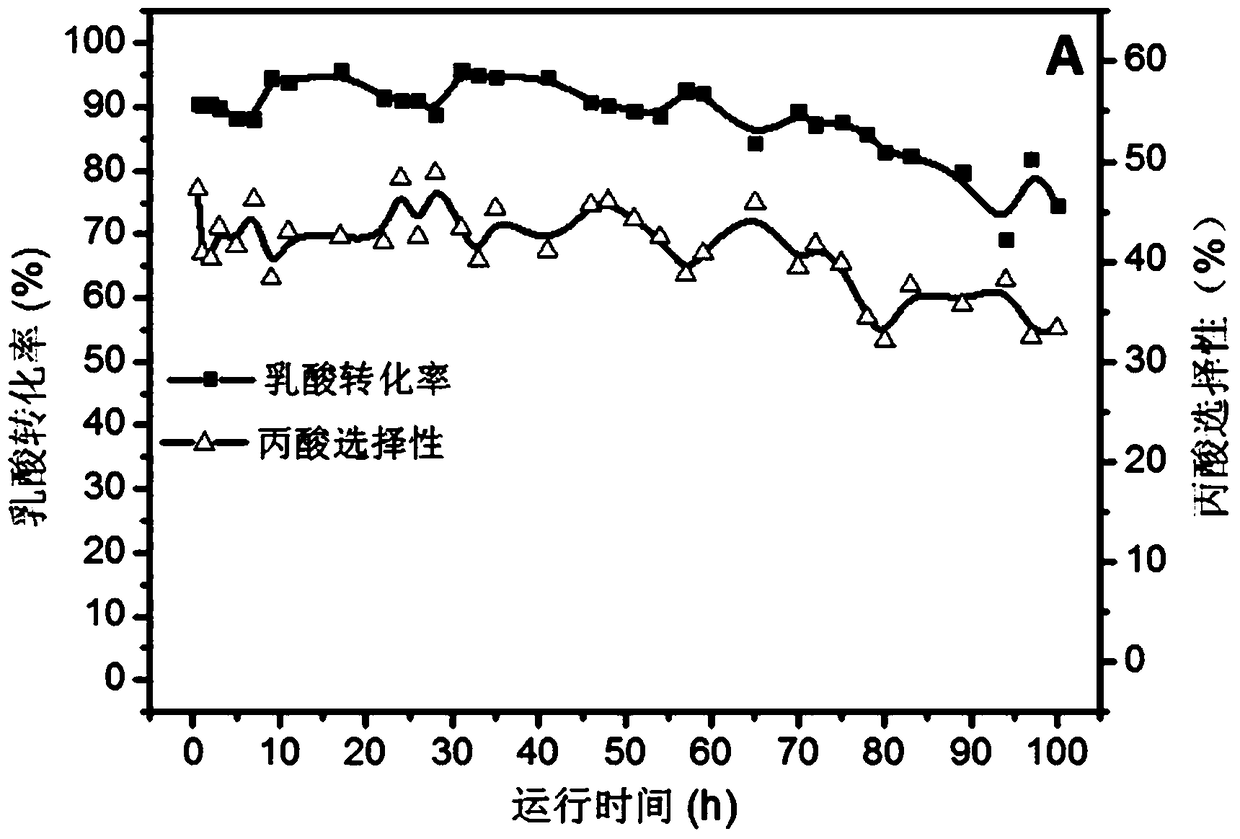
[0032] figure 1 This is a diagram of the pore structure of the catalyst prepared by using ferric nitrate as an iron source in the present invention. The specific experimental steps are as follows: first, weigh 8.08g of nonahydrate ferric nitrate and fully dissolve it in 100ml of deionized water. Use 25-28% ammonia water as a precipitating agent, adjust the pH to about 8-9, continue stirring at room temperature for 1 hour, stop stirring for 2 hours, filter with suction, wash with deionized water for 3-5 times, and dry by blowing Dry at 120℃. In an air atmosphere, the temperature was programmed to 500°C and calcined for 6 hours. The obtained iron oxide is tableted and granulated, and 20-40 mesh is taken as a catalyst for use. A quartz tube with a length of 400mm and an inner diameter of 3mm is selected, and 0.44g of the above-mentioned spare catalyst is filled in the quartz tube. The length of the catalyst in the tube is 30mm. The two ends of the catalyst are terminated with qua...
Embodiment 2
[0034] First, weigh 8.00 g of iron sulfate (without crystal water) and fully dissolve it in 100 ml of deionized water. Use 25-28% ammonia water as a precipitating agent, adjust the pH to about 8-9, continue stirring at room temperature for 1 hour, stop stirring for 2 hours, filter with suction, wash with deionized water for 3-5 times, and dry by blowing Dry at 120℃. In an air atmosphere, the temperature was programmed to 500°C and calcined for 6 hours. The obtained iron oxide is tableted and granulated, and 20-40 mesh is taken as a catalyst for use. A quartz tube with a length of 400mm and an inner diameter of 3mm is selected, and 0.44g of the above-mentioned spare catalyst is filled in the quartz tube. The length of the catalyst in the tube is 30mm. The two ends of the catalyst are terminated with quartz wool. The length of the quartz wool is 30mm. . The aqueous lactic acid solution was carried by nitrogen through the above-mentioned quartz tube filled with the catalyst, th...
Embodiment 3
[0037] First, weigh 8.00 g of iron sulfate (without crystal water) and fully dissolve it in 100 ml of deionized water. Use ethylenediamine as a precipitant, adjust the pH to about 8-9, continue stirring at room temperature for 1h, stop stirring for 2h, filter with suction, wash with deionized water for 3-5 times, and place in a blast drying oven at 120°C. dry. In an air atmosphere, the temperature is programmed to 500°C and calcined for 6 hours. The obtained iron oxide is tableted and granulated, and 20-40 mesh is taken as a catalyst for use. A quartz tube with a length of 400mm and an inner diameter of 3mm is selected, and 0.13g of the above-mentioned spare catalyst is filled in the quartz tube. The length of the catalyst in the tube is 30mm. The two ends of the catalyst are terminated with quartz wool. The length of the quartz wool is 30mm. . The aqueous lactic acid solution was carried by nitrogen through the above-mentioned quartz tube filled with the catalyst, the temper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com