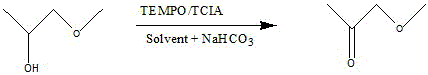Method for synthesizing methoxy acetone
A technology of methoxyacetone and methoxyl group, applied in the field of synthesis of methoxyacetone, can solve the problems of difficult recovery, difficult oxidation of 1-methoxy-2-propanol, difficult product separation, etc., and achieves easy industrialization The effect of mild production and reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] In the 1000ml four-necked reaction flask (with mechanical stirring, thermometer, dropping funnel and reflux condenser), add 90 grams of 1-methoxyl-2-propanol, then add 360 grams of dichloromethane, then add 0.47 grams of TEMPO , then add 96.6 grams of sodium bicarbonate, lower the temperature to 0-10°C, and gradually add 81.4 grams of oxidant TCIA. During this process, use an ice-salt bath to cool down, so that the temperature is maintained at 0-10°C, and the addition is completed within 3 hours. Afterwards, the reaction was continued for 0.5 hours, and samples were taken for gas chromatography (GC) analysis of raw material content (<0.5%). After the reaction was completed, the reaction solution was filtered, and the filter cake was washed with 100 grams of dichloromethane. After the mother liquor and washing liquid were combined, they were concentrated, the dichloromethane was recovered, and then vacuum distillation was carried out to obtain methoxyacetone: 86.1 grams, ...
Embodiment 2
[0016] In the 1000ml four-necked reaction flask (with mechanical stirring, thermometer, dropping funnel and reflux condenser), add 90 grams of 1-methoxyl-2-propanol, then add 400 grams of dichloromethane, then add 0.47 grams of TEMPO , then add 96.6 grams of sodium bicarbonate, lower the temperature to 10-20°C, and gradually add 81.4 grams of oxidizing agent TCIA. During this process, use an ice-salt bath to cool down, keep the temperature at 10-20°C, and finish adding within 3 hours. The reaction was continued for 0.5 hours, and a sample was taken for GC analysis of the raw material content (<0.5%). After the reaction, the reaction solution was filtered, and the filter cake was washed with 100 grams of dichloromethane. After the mother liquor and washing liquid were combined, they were concentrated, and the dichloromethane was recovered, and then vacuum distillation was carried out to obtain methoxyacetone: 85.5 grams, GC content: 99.7%, yield: 97.2%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap