Method for preparing novel and efficient reduced ion particle fillers
A particle filler, reduced iron technology, applied in chemical instruments and methods, water pollutants, water/sewage treatment, etc., can solve the problems of decreased iron reaction activity, no obvious improvement in the passivation of the surface of the filler, etc., and achieves high catalytic efficiency. The effect of reducing the cost of firing and overcoming the passivation of the filler surface
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
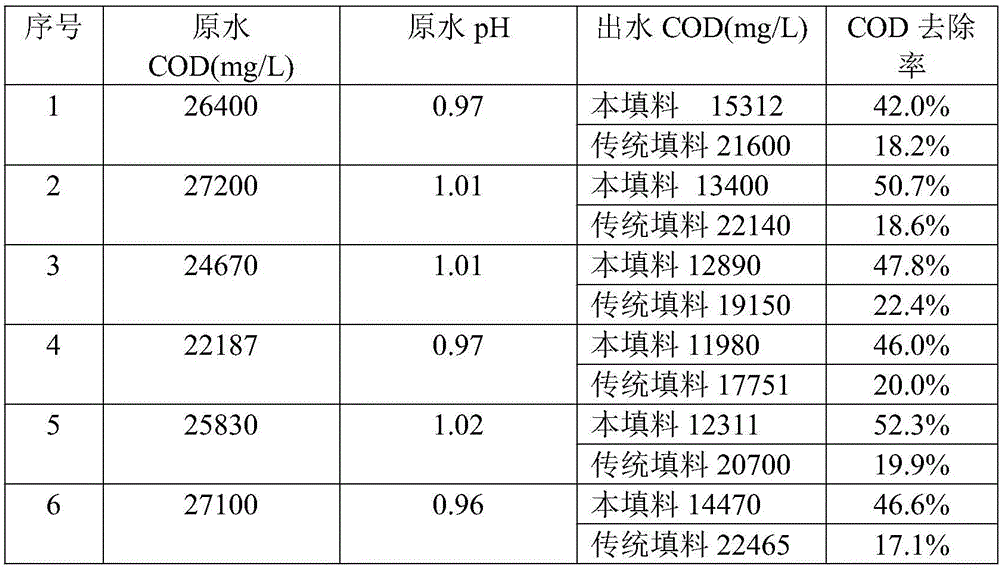
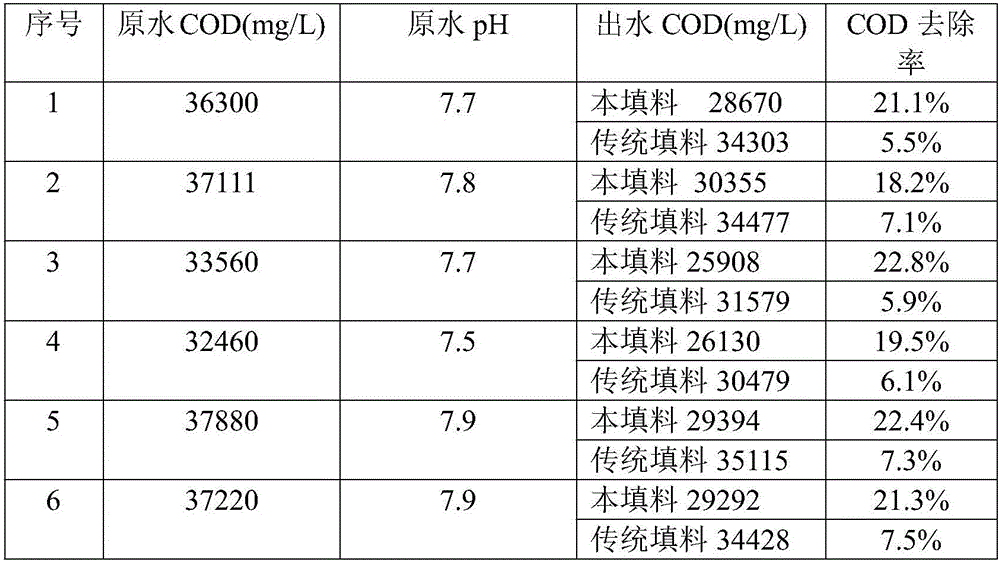
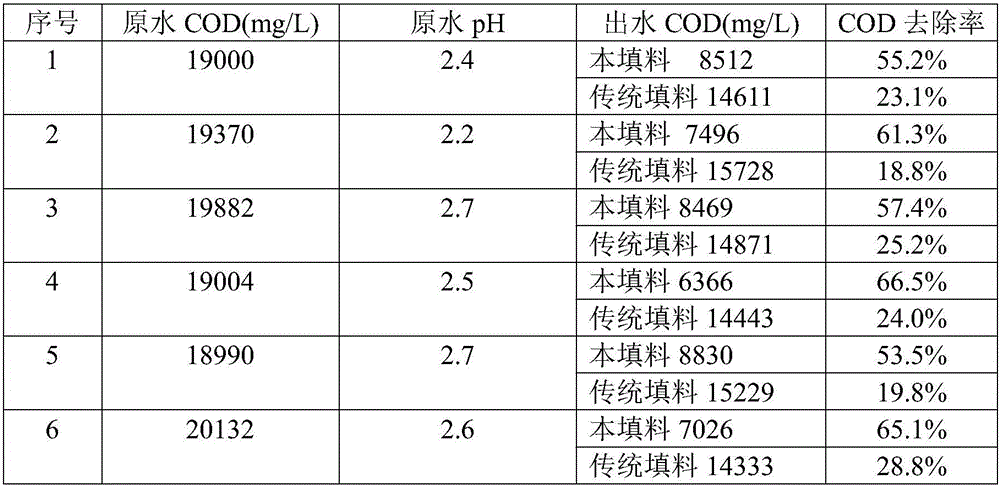
[0024] Get iron powder, pass 100 mesh sieves. Take manganese dioxide, pulverize and grind, and pass through a 100-mesh sieve. Take graphite, crush and grind it, and pass it through a 100-mesh sieve; respectively take zinc powder and zinc chloride, crush and grind it, pass it through a 100-mesh sieve, and mix them thoroughly at a weight ratio of 1:3. The sieved iron powder, manganese dioxide, graphite powder, zinc powder and zinc chloride mixture are fully mixed according to the mass ratio of 80:10:5:1 to obtain the mixture A; respectively take copper powder and copper chloride, pulverize Grind, pass through a 100-mesh sieve, and fully mix with a weight ratio of 8:1 to make material B. Mix mixture A and material B at a mass ratio of 10:1, and evenly spray 8% potassium hydroxide solution into the mixture according to the mass ratio of mixture to potassium hydroxide solution of 12:1, shape, dry, fill in cans, and enter For the kiln, heat up to 300°C for 1 hour without air; then...
Embodiment 2
[0026] Get iron powder, pass 100 mesh sieves. Take manganese dioxide, pulverize and grind, and pass through a 100-mesh sieve. Take graphite, crush and grind it, and pass it through a 100-mesh sieve; respectively take zinc powder and zinc chloride, crush and grind it, pass it through a 100-mesh sieve, and mix them thoroughly at a weight ratio of 1:3. The sieved iron powder, manganese dioxide, graphite powder, zinc powder and zinc chloride mixture are fully mixed according to the mass ratio of 80:15:5:2 to obtain the mixture A, respectively take copper powder and copper chloride, pulverize Grind, pass through a 100-mesh sieve, and mix thoroughly at a weight ratio of 8:1 to obtain material B; mix material A and material B at a mass ratio of 14:1, and mix material and potassium hydroxide solution at a mass ratio of 12:1 Spray 8% potassium hydroxide solution evenly into the mixture, shape, dry, put in cans, put into kiln, then heat up to 300°C for 1 hour without air; then heat up ...
Embodiment 3
[0028] Get iron powder, pass 100 mesh sieves. Take manganese dioxide, pulverize and grind, and pass through a 100-mesh sieve. Take graphite, crush and grind it, and pass it through a 100-mesh sieve; respectively take zinc powder and zinc chloride, crush and grind it, pass it through a 100-mesh sieve, and mix them thoroughly at a weight ratio of 1:3. The sieved iron powder, manganese dioxide, graphite powder, zinc powder and zinc chloride mixture are fully mixed according to the mass ratio of 80:15:5:1 to obtain the mixture A; respectively take copper powder and copper chloride, pulverize Grind, pass through a 100-mesh sieve, and mix thoroughly at a weight ratio of 8:1 to make material B. Mix mixture A and material B at a mass ratio of 12:1, and mix the mixture to potassium hydroxide solution at a mass ratio of 12:1 Spray 8% potassium hydroxide solution evenly into the mixture, shape it, dry it, put it in cans, and put it into a kiln. Then the temperature was raised to 300°C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com