Method for screening alpha-amylase inhibitor
An amylase inhibitor and amylase technology, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of unclear active ingredients, difficult screening, time-consuming and laborious, etc., and achieve intuitive screening results, fast screening, Effect with high sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Solution preparation
[0035] Preparation of enzymatic reaction buffer: Dissolve 302.5mg tris and 330mg anhydrous calcium chloride in water, adjust the pH to 7.5 with hydrochloric acid, and then add water to make the volume up to 100mL, and the pH is 7.5, three The enzymatic reaction buffer with a molar concentration of hydroxymethylaminomethane of 25 mmol / L is stored in a refrigerator at 2 to 4°C for later use.
[0036] Preparation of substrate solution: 312mg NaH 2 PO 4 ·2H 2 O and 716mg Na 2 HPO 4 ·12H 2 Dissolve O in 100mL of deionized water and adjust the pH to 6.9 to obtain a phosphate buffer solution with a pH of 6.9 and a phosphate concentration of 20mmol / L; take 1g of soluble starch and dissolve it in 100mL of the above phosphate buffer solution and place it in a 95℃ water bath After heating for 4 hours, let it cool and centrifuge at 8000 rpm for 10 minutes. The supernatant obtained is the substrate solution of phosphate buffer solution containing 1wt% soluble st...
Embodiment 2
[0043] The difference between this embodiment and embodiment 1 is that the substrate solution used is a phosphate buffer solution with a pH of 6.9 and containing 0.05 wt% soluble starch and a phosphoric acid with a pH of 6.9 and 1 wt% soluble starch. The salt buffer solution, and the rest of the solutions are the same as described in Example 1.
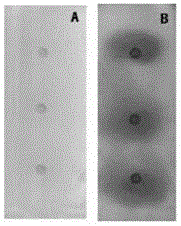
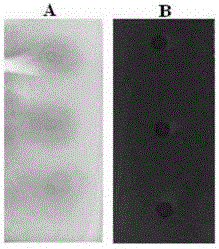
[0044] Spot the test solution on two thin-layer plates (abbreviated as: A plate and B plate), and each plate was spotted at 3 points in parallel, and the amount of sample was 1 μL; the methanol solvent was evaporated and immersed in In the above substrate solution; take out, evaporate the solvent, and then immerse in the α-amylase solution, carry out the enzymatic reaction at 37°C for 30 minutes; after the reaction is completed, take out the thin-layer plate, evaporate the solvent, and then immerse in the color development Color reaction in the reagent solution; inspection under visible light, the inspection result is as figure 2 Shown:...
Embodiment 3
[0046] This embodiment is different from Embodiment 1 only in that the iodine content in the developer solution used is 0.1 mg / mL and 20 mg / mL, respectively, and the rest of the solutions are the same as those described in Embodiment 1.
[0047] Spot the test solution on two thin-layer plates (abbreviated as: A plate and B plate), and each plate was spotted at 3 points in parallel, and the amount of sample was 1 μL; the methanol solvent was evaporated and immersed in In the substrate solution; remove and evaporate the solvent, then immerse in the α-amylase solution, and carry out the enzymatic reaction at 37°C for 30 minutes; after the reaction is completed, remove the thin-layer plate, evaporate the solvent, and then immerse in the above-mentioned color development Color reaction in the reagent solution; inspection under visible light, the inspection result is as image 3 Shown: all sample points of the test solution on the A and B plates have blue spots on a white or yellow or b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com