Immuno-chromatography kit for quickly and quantitatively detecting calprotectin in excrement
A calprotectin, quantitative detection technology, applied in biological testing, measuring devices, analytical materials, etc., can solve the problems of unsuitable clinical rapid diagnosis, high requirements for testing equipment, poor repeatability, etc., to achieve reliable diagnosis results, improve The effect of detection stability and improved detection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Composition of the Immunochromatographic Kit for Quantitative Detection of Fecal Calprotectin
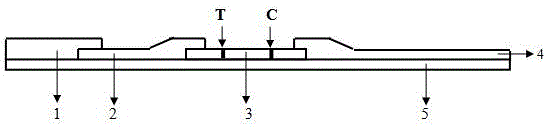
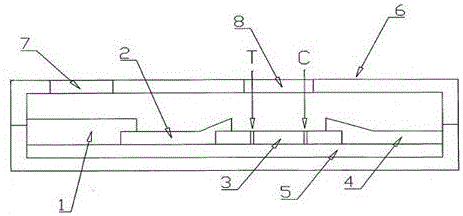
[0032] combine Figure 1-3 Describe the structure of the immunochromatographic kit for the quantitative detection of fecal calprotectin. The immunochromatography kit includes a test strip, and the test strip includes a base plate 5, a sample pad 1 arranged on the base plate 5, a marker binding pad 2, a nitrocellulose membrane 3 and an absorbent pad 4, and the marker binding pad 2 packs Calprotectin monoclonal antibody A is labeled with colloidal gold particles, and the nitrocellulose membrane 3 contains parallel detection line T and quality control line C. The detection line T is formed by calprotectin monoclonal antibody B, and the quality control line C is formed by Goat anti-mouse IgG was formed, and calprotectin monoclonal antibodies A and B recognized different epitopes of calprotectin.
[0033] Calprotectin monoclonal antibody A was purchased from Zhuhai Bomei...
Embodiment 2
[0038] Example 2 Preparation of an immunochromatographic kit for quantitative detection of fecal calprotectin
[0039] In Example 1, the kit was prepared by the following method:
[0040] 1. Sample pad
[0041] The glass cellulose membrane was soaked in a surfactant buffer solution and dried at 37°C and a relative humidity of less than 20% to obtain a sample pad. The surfactant buffer solution is to add casein with a final concentration of 5% (mass percentage concentration), 5% (mass percentage concentration) of Polyvinylpyrrolidone 10, 1% (mass percentage concentration) in pH9.0 boric acid buffer solution It is formed after sodium cholate, 1.8% (mass percentage concentration) of RHODASURF ON-870 (SIGMA company reagent) and 0.02% (mass percentage concentration) sodium azide.
[0042] pH9.0 boric acid buffer solution: add 980 ml of ultrapure water to 6.18 g of boric acid to dissolve, then adjust the pH to 9.0 with 10 mol / L NaOH aqueous solution, and add ultrapure water to 100...
Embodiment 3
[0053] Example 3 The use method and effect of the immunochromatographic kit for quantitative detection of fecal calprotectin
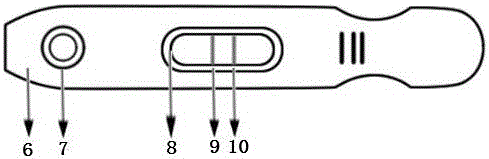
[0054] 200 clinical stool samples were sampled with a stool specimen box, diluted and mixed with normal saline. In the sample hole 7 of the kit in Example 1, 3-4 drops of the treated sample were added dropwise, and after 10 minutes, the detection line T and the quality control line C were analyzed by an intelligent immune quantitative analyzer or an automatic stool analyzer. Optical density, calculate the concentration of fecal calprotectin in the processed sample according to the standard curve preset in the quantitative analyzer, the unit is ng / ml. Calculate the mass of fecal calprotectin per gram of fecal sample in ug / g.
[0055] The above-mentioned 200 clinical samples were detected by using the fecal calprotectin detection kit (ELISA) produced by Buhlmann Laboratories AG of Switzerland at the same time, and the correlation of the detection result...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com