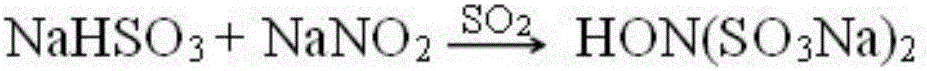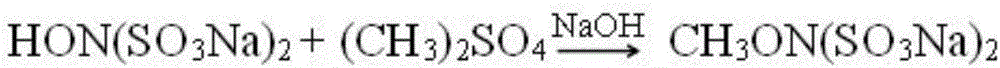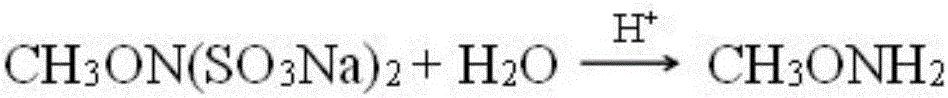Method for coproducing vasoxine hydrochloride and N,O-dimethylhydroxylamine hydrochloride
A technology of dimethylhydroxylamine hydrochloride and methoxyamine hydrochloride, applied in the direction of organic chemistry and the like, can solve the problems of ineffective treatment of salty wastewater, serious environmental protection problems, etc., and achieves excellent product quality, low comprehensive cost, Simple and reliable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] In a 1000mL four-neck flask reactor equipped with a thermometer, a stirrer, a constant pressure funnel, and an exhaust gas water seal, add 69 g (1 moL) of hydroxylamine hydrochloride and 69 g of water, and add 140 g of 30% NaOH (1.05 moL) dropwise at 20 to 25 ° C, Add 53g Na 2 CO 3 (0.5moL), add CH evenly within 8h 3 Cl 150.5g (3moL), while adding 30% NaOH dropwise to adjust the pH=9.5-10, then keep stirring at 2-5°C for 2h.
[0044] Change the flask to a distillation method, add 30% NaOH dropwise to adjust the pH=12, heat up and distill for 2 to 8 hours, collect the condensate with a vapor phase temperature of 60-100°C to obtain the stripping fraction, and reduce the raffinate at 75°C (vacuum-0.075MPa). Concentrate under high pressure until a large amount of salt precipitates out, filter while hot to obtain sodium chloride as a by-product, and add the mother liquor back to the next batch of raffinate to continue concentrating.
[0045] In a packed column equipped wi...
Embodiment 2
[0050] The hydroxylamine salt is changed to hydroxylamine sulfate (1mol), and the methylating reagent is changed to (CH 3 ) 2 SO 4 (2.5mol), water 160g, what reclaimed during this moment mother liquor neutralization and distillation is sodium sulfate, other is with embodiment 1. After applying the mother liquor mechanically for 5 times, the calculated total average yield of methoxyamine hydrochloride was 39%, and that of N,O-dimethylhydroxylamine hydrochloride was 31%.
Embodiment 3
[0052] The addition amount of hydroxylamine salt and methylating agent is changed into: CH of 1moL hydroxylamine hydrochloride and 1.5moL 3 Cl, and CH 3Cl was added evenly within 25 hours, and at the same time, alkali was added to adjust the pH to 11-12, and then the reaction was carried out at 35-40°C with stirring for 4 hours. Add 31% hydrochloric acid to N,O-dimethylhydroxylamine fraction to adjust pH = 2 to form a salt, add 31% hydrochloric acid to adjust pH = 2 to form a salt, both products are concentrated and crystallized under reduced pressure at 85°C, pumped after cooling Filter, recrystallize the filter cake with methanol, and dry it in vacuum at 60°C. Others are the same as in Example 1. After applying the mother liquor 5 times, the total average yield of methoxyamine hydrochloride is 50%, and N,O-dimethylhydroxylamine salt Salt is 11%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com