Application of miRNA-489 to preparation of medicines for treating silicosis
A technology of mirna-489, 1. mirna-489 is applied in the application field of miRNA-489 in the preparation of silicosis drugs, and achieves the effects of quantitative accuracy, improving affinity and increasing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Reconstruction of mouse silicosis model
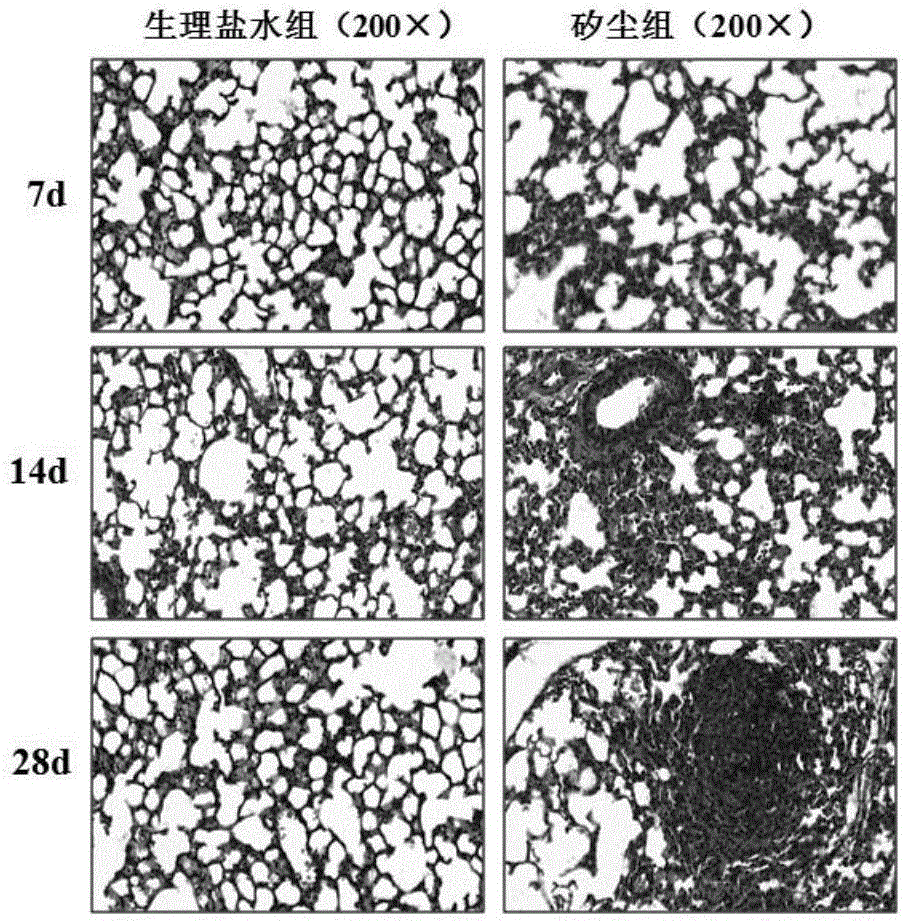
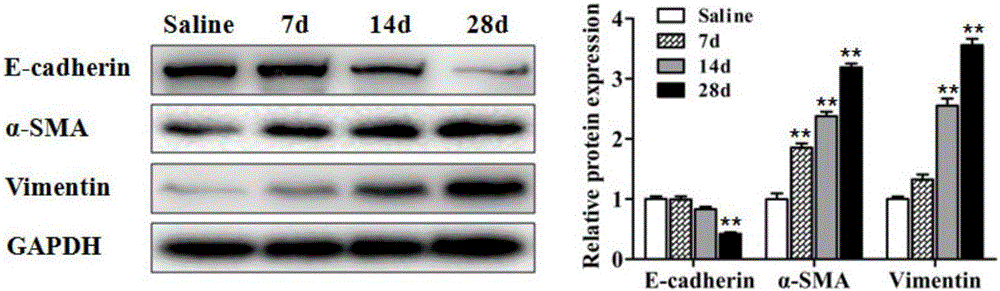
[0037] Establishment of mouse lung fibrosis animal model induced by silica dust: Select male C57BL / 6 mice with a body weight of 20-25g for 6-8 weeks, and perfuse 50mg / mL SiO2 prepared by 50μL normal saline through the bronchus under anesthesia. 2 The dust suspension, the control group was given normal saline, the mice were treated on the 7th, 14th, and 28th day, and the lung tissue was preserved. The right lower lung lobe was fixed in formaldehyde, embedded in paraffin, and sectioned for HE staining. The remaining lung tissues were quickly frozen in liquid nitrogen and stored in a -80°C refrigerator for later use. The protein levels of epithelial cell markers (E-cadherin) and mesenchymal cell markers (α-SMA, Vimentin) in lung tissue were determined by western blot. The results of mouse lung tissue pathological sections and fibrosis indicators were combined to evaluate whether the model of silica dust-induced lung fi...
Embodiment 2
[0047] Example 2: Reconstructing the qRT-PCR experiment of miRNA-489 levels in the mouse silicosis model
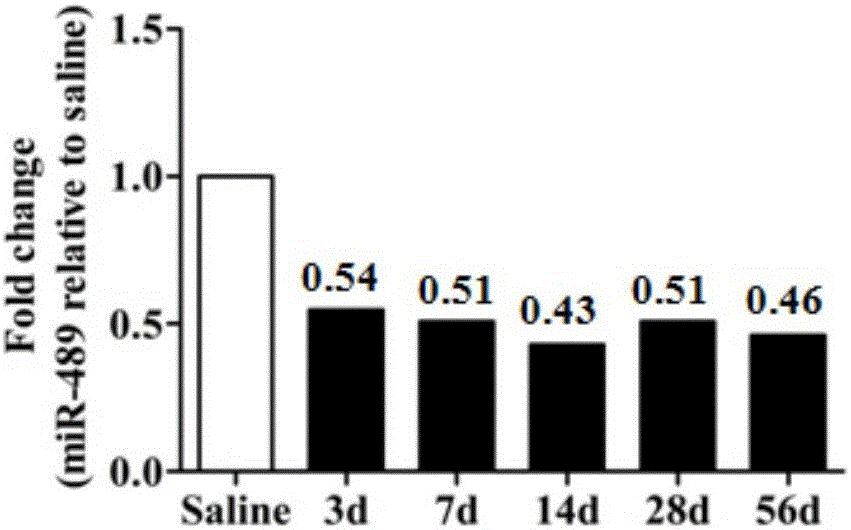
[0048] After confirming that the model was successfully established, RNA was extracted from the lung tissue of 5 mice in each of the 7th, 14th, and 28th d silica dust treatment groups and 28 d saline group according to the pathological sections of the lung tissue for qRT-PCR detection. Strict quality control was implemented throughout the research process, each sample was tested three times in a row, and all samples were blinded, that is, completed without knowing the background of the sample to avoid bias.
[0049] (1) Preparation of RNA samples
[0050]Place the sterilized tissue homogenizer on ice, add 100 mg of mouse lung tissue, and then add 1.0 mL Trizol (Life Technologies / ambion, Carlsbad, CA) to grind until homogenized; ② Transfer the homogenate to 1.5 mL Add 200 μL of chloroform (trichloromethane) to the RNase EP tube, sandwich it between two plates and gently i...
Embodiment 3
[0055] Example 3: Animal experiment of silica dust-induced pulmonary fibrosis in mice by up-regulating the level of miRNA-489
[0056] (1) Animal model establishment
[0057] A chemically modified miRNA-489 agonist (agomir) was used to upregulate miRNA-489 levels in mice. Select male C57BL / 6 mice with a body weight of 20-25g for 6-8 weeks, and divide the mice into control group (normal saline), silica dust group (SiO 2 suspension), miR-NC+silica dust group (miR-NC+SiO 2 suspension), miRNA-489 high expression + silica dust group (miRNA-489agomir+SiO 2 Suspension), 50mg / mL SiO prepared by perfusing 50μL normal saline through the bronchus under anesthesia 2 Dust suspension, in which the miRNA-489 high expression group was perfused with SiO 2 After the dust suspension, 5 nmol miRNA-489 agomir was injected into the trachea for the first time, and then 2.5 nmol miRNA-489 agomir was injected through the tail vein on 7, 14 and 21 days respectively, and lung tissue and serum were c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com