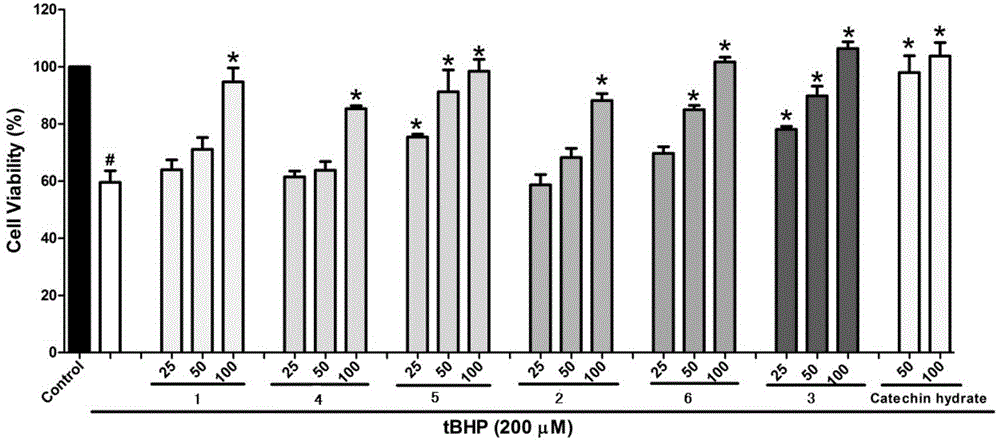
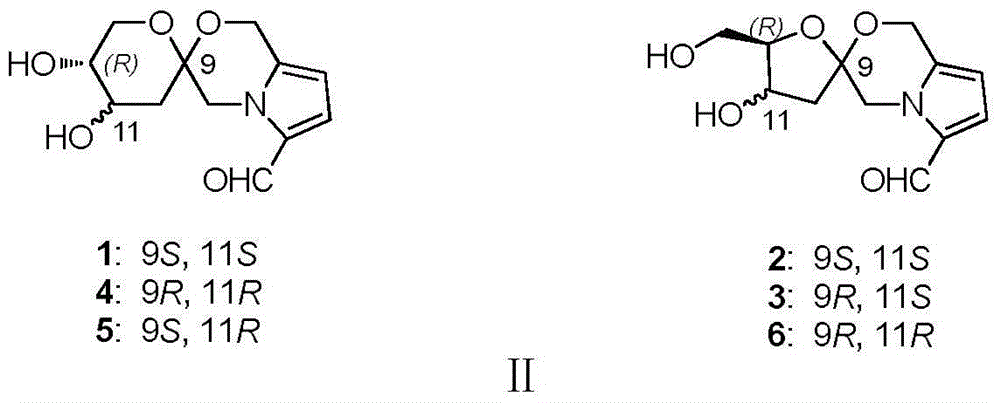
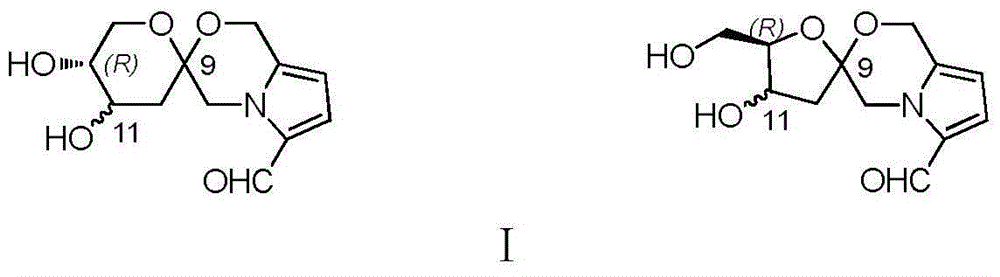Pyrrole and morpholine spirocyclic alkaloid compound and preparation method and application thereof
A technology of pyrrolomorpholine spiro and rolomorpholine spiro, which is applied in the field of pharmacy and can solve problems such as oxidative stress, disruption of oxygen free radical homeostasis, damage to antioxidant defense system, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 extracts and isolates compounds 1, 2, and 3 by Wuling powder
[0049] 20kg of Wuling bacteria powder (Xylaria nigripes) was sequentially extracted 3 times with 20L of 75% ethanol for 36 hours each time, and the obtained extracts were combined and concentrated under reduced pressure at 65°C to obtain 1.3kg of extracts, which were dispersed with 1L of water, followed by Extract with petroleum ether (3×1L) and ethyl acetate (4×2L); after concentrating under reduced pressure, obtain 70.5g of petroleum ether part extract and 281.6g of ethyl acetate part extract respectively, use silica gel For column chromatography, the ethyl acetate part was eluted through a normal phase silica gel column, and PE: EA (20:1-2:1), CH 2 Cl 2 : MeOH (50:0–0:1) gradient elution to obtain 15 components (Fr.1-Fr.15). Fr.11f (300.0mg) was purified by silica gel column chromatography to CH 2 Cl 2 : MeOH (50:1)) after elution by Sephadex LH-20 (MeOH, CH 2 Cl 2 : MeOH, 2:1) was puri...
Embodiment 2
[0051] Step 1, synthesis of (S)-1-((R)-2,2-dimethyl-1,3-dioxolan-4-yl)but-3-en-1-ol
[0052] Dissolve 5g (38.5mmol) of compound 7 in dry tetrahydrofuran (35ml), cool down to -78°C, slowly add 34ml (57.8mmol) of 1.7M allylmagnesium chloride dropwise, and keep the temperature below -70°C during the dropwise addition After the addition, continue to react at this temperature for 2 hours, slowly add 30ml of saturated ammonium chloride solution at low temperature to quench the reaction, dilute with water, extract the aqueous phase with ethyl acetate for 3 times, combine the organic phases and wash once with saturated sodium chloride , dried over anhydrous magnesium sulfate, filtered, and concentrated. The crude product was purified by silica gel column chromatography to obtain 5.7 g of colorless liquid (8), with a yield of 86.0%.
[0053] 1 H NMR (400MHz, CDCl 3 ):δ8a:5.85(m),5.18(m),5.13(m),4.03(m),3.94(m),3.78(m),2.34(m),2.23(m),1.44(s),1.37 (s); 8b: δ5.85(m), 5.15(m), 4.03(m),...
Embodiment 3
[0069] Step 1, the operation of synthesizing (S)-1-((R)-2,2-dimethyl-1,3-dioxolan-4-yl)but-3-en-1-ol compound 8 and The operation of embodiment 1 is the same, and the reaction reagent allylmagnesium chloride is changed into allylmagnesium bromide, and the yield is 80%
[0070] Step 2, synthesis of tert-butyl (((S)-1-((R)-2,2-dimethyl-1,3-dioxolan-4-yl)but-3-en-1-yl )Oxy)dimethylsilane
[0071] Take 1g (5.8mmol) of compound 8, dissolve it in dry dichloromethane (10ml), fill it with nitrogen protection, after cooling in an ice-water bath, add triethylamine (1.2g) and tert-butyldimethylsilyl chloride ( 1.1g), after the addition, slowly rise to room temperature and react for 16h, add water to quench the reaction, wash once with 1M potassium bisulfate, extract the aqueous phase twice with dichloromethane, wash the combined organic phase once with saturated sodium chloride, and wash with anhydrous sulfuric acid Dried over magnesium, filtered and concentrated, the crude product was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com