Quality standard for Dieda analgesic ointment and testing method thereof
A Dieda analgesic ointment, quality standard technology, applied in the field of medicine, can solve the problems of ineffective monitoring of preparations, inability to fully ensure the efficacy of preparations, etc., and achieve high accuracy, good linear relationship, and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Implementation method of microscopic identification of embodiment 1 Dieda analgesic ointment
[0045]Take Dieda analgesic ointment, remove the cover lining, cut it into pieces, take a small amount of ointment, and observe it under a microscope: the fiber bundles are bright yellow, and the surrounding cells contain square crystals of calcium oxalate, forming crystal fibers, and the cell walls containing crystals are lignified and thickened ( Treats). Fuchsia fibers and crystal fibers are bundled or scattered individually, the walls are thick, and are evenly wooded (Dalbergia). Bast fiber light yellow, fusiform, thick wall, fine pores and grooves (Scutellaria baicalensis). Calcium oxalate clusters are large, with a diameter of 20-140 μm (rhubarb). Single-celled nonglandular hairs resemble fibers, mostly broken, and the base is enlarged like stone cells, lignified (nychnium). Body wall fragments are yellow or brownish red, with round hairy nests, 8-24 μm in diameter, an...
Embodiment 2
[0046] The implementation method of volatile oil identification in embodiment 2 Dieda analgesic ointment
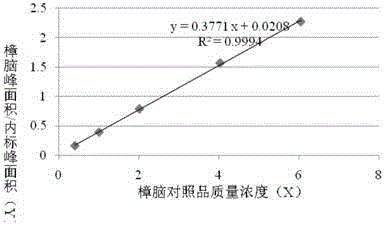
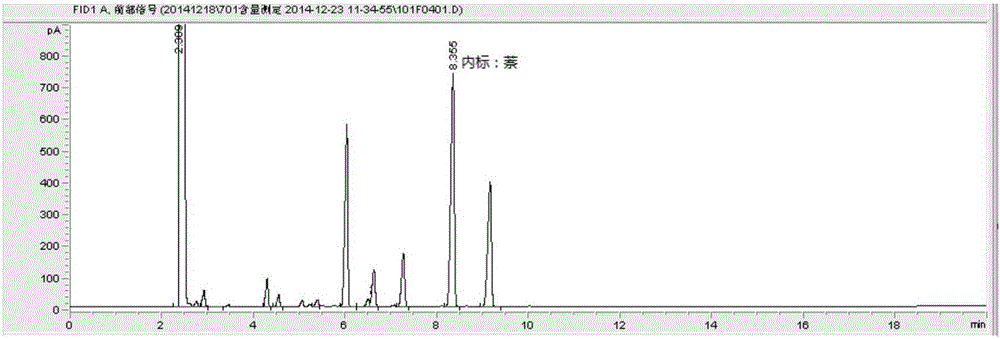
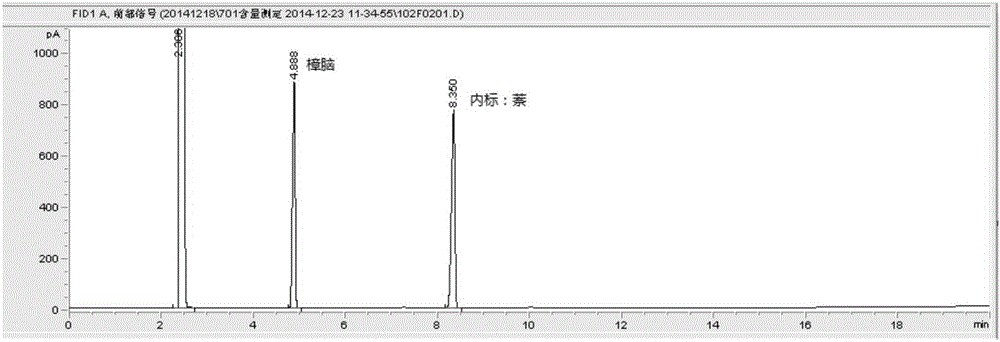
[0047] Extract according to method A of volatile oil determination method (2015 edition pharmacopoeia general rule 2204 method A), in the liquid in volatile oil measuring device, add ethyl acetate 10ml and shake and extract, divide and get ethyl acetate liquid, as need testing solution; Reference substance, borneol reference substance, methyl salicylate reference substance and camphor reference substance, according to gas chromatography (2015 edition pharmacopoeia general rule 0521) test, draw reference substance solution and need testing solution respectively, inject gas chromatograph, for testing The chromatographic peaks with the same retention time as the chromatographic peaks of the reference substance should appear in the chromatogram of the product.
Embodiment 3
[0048] The qualitative examination method of strychnine in embodiment 3 Dieda analgesic ointment
[0049] Take 5 tablets of Dieda Analgesic Ointment, remove the lid lining, cut into small pieces, put them in a stoppered Erlenmeyer flask, add 60ml of 1mol / L hydrochloric acid solution, soak overnight, ultrasonicate for 20 minutes, filter, and put the filtrate in a separating funnel In the medium, add concentrated ammonia test solution to adjust the pH value to 9-10, shake and extract 3 times with ether (30ml, 20ml, 20ml), combine the ether solution, add anhydrous sodium sulfate to dehydrate, filter, evaporate the filtrate to dryness, and add the residue to Chloroform was dissolved, moved to a 2ml measuring bottle, added chloroform to dilute to the mark, shaken up, and used as the test solution; another appropriate amount of strychnine reference substance was taken, accurately weighed, added chloroform to make each 1ml of the solution containing 6mg is used as the reference subst...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com