Topiroxostat controlled-release capsule and preparation method thereof
A technology of topicastat and capsules, which is applied in the field of topicastat controlled-release capsules and their preparation, can solve problems such as obvious side effects, and achieve the effects of improving bioavailability, preventing burst release, and being easy to purchase.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
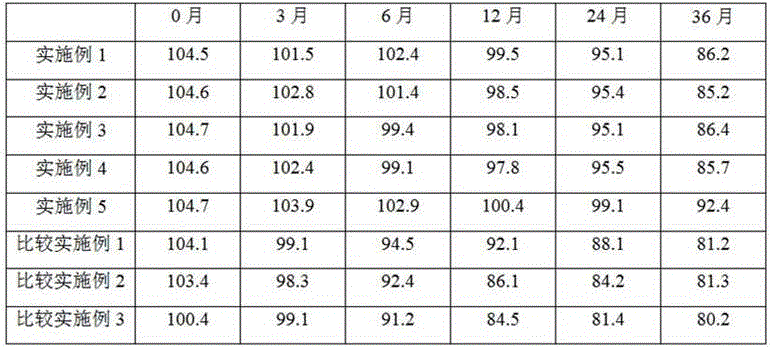
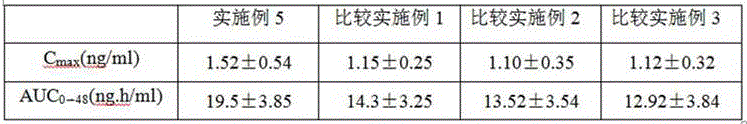
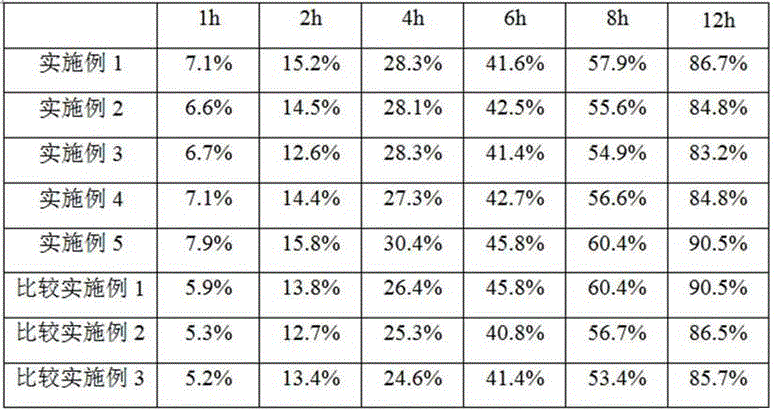
Examples
Embodiment 1
[0040] Topicastat 27.5g
[0041] β-cyclodextrin 106g
[0042] Ethylcellulose 6.62g
[0043] Sodium carboxymethylcellulose 0.66g
[0044] Surelease E-7-19040 21.33g
[0045] Hypromellose E5 0.26g
[0046] Appropriate amount of purified water
[0047] The specific preparation method is as follows:
[0048] (1) Take an appropriate amount of purified water and heat it to 80°C, add β-cyclodextrin and stir to dissolve, then add topinostat, and stir for 30 minutes to form a topinostat solution;
[0049] (2) Adjust the air inlet temperature of the spray drier to 160° C., the air outlet temperature to 80° C., and the pressure to 0.3 MPa. The above-mentioned topinostat solution is spray-dried to obtain the topinostat soluble powder;
[0050] (3) Get 66.25g of above-mentioned topinastat soluble powder, ethyl cellulose, sodium carboxymethyl cellulose and mix, add 26.47g of water, mix and make soft material;
[0051] (4) Centrifuge-fluidized granulation coating method to granulate, ...
Embodiment 2
[0055] Topicastat 27.5g
[0056] β-cyclodextrin 106g
[0057] Ethylcellulose 6.62g
[0058] Sodium carboxymethylcellulose 0.662g
[0059] Surelease E-7-19040 17.07g
[0060] Hypromellose E5 0.21g
[0061] Appropriate amount of purified water
[0062] The specific preparation method is as in Example 1.
Embodiment 3
[0064] Topicastat 27.5g
[0065] β-cyclodextrin 96g
[0066] Ethylcellulose 8.31g
[0067] Sodium carboxymethylcellulose 0.332g
[0068] Surelease E-7-19040 24.81g
[0069] Hypromellose E5 0.49g
[0070] Appropriate amount of purified water
[0071] The specific preparation method is as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com