Application of carher1-nkt cells in the preparation of preparations for the treatment of advanced HER1-positive cholangiocarcinoma
A technology of NKT cells and cholangiocarcinoma, applied in gene therapy, cells modified by introducing foreign genetic material, drug combinations, etc., can solve the problems of not improving the survival rate of patients, affecting the efficacy of drugs, and drug resistance of patients, so as to avoid Effects of drug resistance, enhanced specific killing activity, enhanced ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] In the application of the present invention, preferably, the preparation method of CARHER1-NKT cells includes: packaging the lentivirus carrying pWPXL-HER1ScFv-CD8-CD137-CD3ζ to obtain a virus concentrate; using the obtained virus concentrate to infect NKT cells, so that NKT cells express the chimeric antigen receptor HER1ScFv-CD8-CD137-CD3ζ, wherein the pWPXL-HER1ScFv-CD8-CD137-CD3ζ is expressed by a lentivirus containing a gene encoding the chimeric antigen receptor HER1ScFv-CD8-CD137-CD3ζ carrier.
[0021] In the application of the present invention, the lentiviral expression vector pWPXL-HER1ScFv-CD8-CD137-CD3ζ can co-transfect packaging cells such as 293T packaging cells with an auxiliary vector to obtain virus concentrates and CARHER1-NKT cells.
[0022] The preparation method of the lentiviral expression vector pWPXL-HER1ScFv-CD8-CD137-CD3ζ is not particularly limited, and can be various methods that can be imagined by those skilled in the art. Preferably, the le...
Embodiment 1
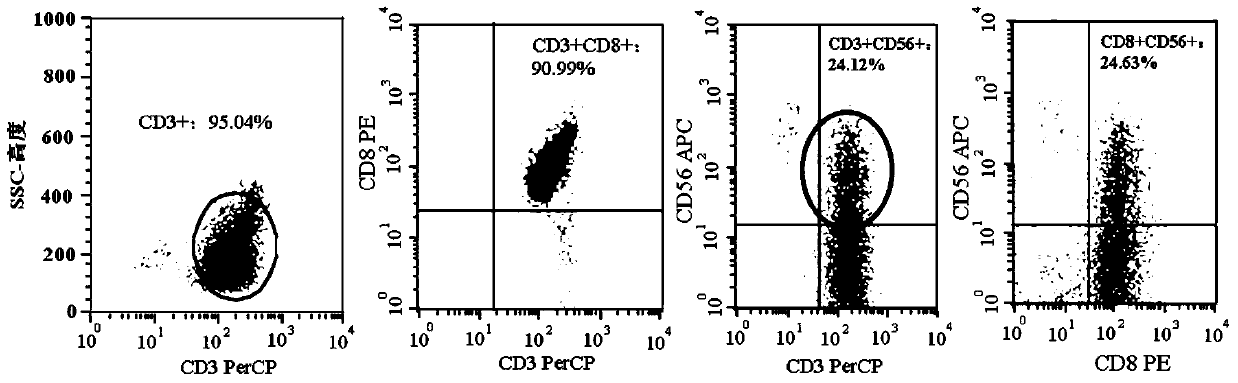
[0074] Example 1 Preparation of NKT cells
[0075] (1) Take human venous blood in a vacuum tube containing heparin. Mononuclear cells (PBMCs) were obtained by density gradient centrifugation using lymphocyte separation medium.
[0076] (2) After PBMCs were washed three times, the final concentration of cells was adjusted to 2×10 by using NKT cell culture medium GT-T551 containing 0.6 volume % human autologous serum. 6 cells / mL; the cells were inoculated on a 75 cm 2 in cell culture flasks. Then add recombinant human interleukin-2 with a final concentration of 500U / mL, 50ng / ml CD3 monoclonal antibody and 50ng / mL recombinant human interleukin-15 to the culture medium, at 37°C and saturated humidity of 5% CO 2 cultured in an incubator.
[0077] (3) On the 4th day of culture, transfer the cells to an uncoated culture flask, add NKT cell culture medium GT-T551 every 2 days according to the number of cell growth, and control the cell concentration to 1×10 8 cells / mL, and added ...
Embodiment 2
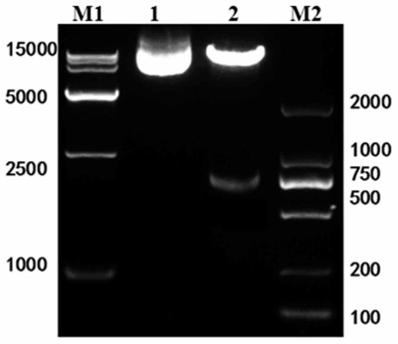
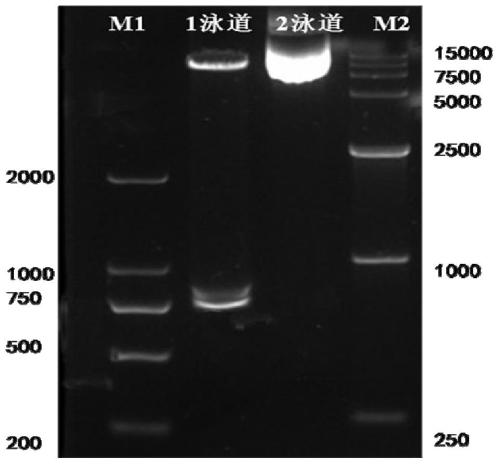
[0078] Example 2 Construction of lentiviral expression vector pWPXL-HER1ScFv-CD8-CD137-CD3ζ
[0079] (1) Preparation of NKT cell cDNA
[0080] The NKT cells cultured in Example 1 were centrifuged to precipitate, and the total RNA of the cells was extracted with RNAiso Reagent, a total RNA extraction kit, and stored at -80°C for future use. Extracted total RNA with RevertAid Reverse Transcription Kit TM NKT cell cDNA was obtained by reverse transcription with the First StrandcDNA Synthesis Kit, and stored at -20°C for future use.
[0081] (2) Preparation of lentiviral plasmid pWPXL-CD8-CD137-CD3ζ
[0082] The following primer sequences were designed and synthesized (wherein, the underline marks are protected bases, and the square boxes are enzyme cleavage sites):
[0083]
[0084] Using the NKT cell cDNA in step (1) as a template, PCR amplification was carried out with primers P1 and P2 to obtain the hinge region and transmembrane region of CD8 with a length of 287bp. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com