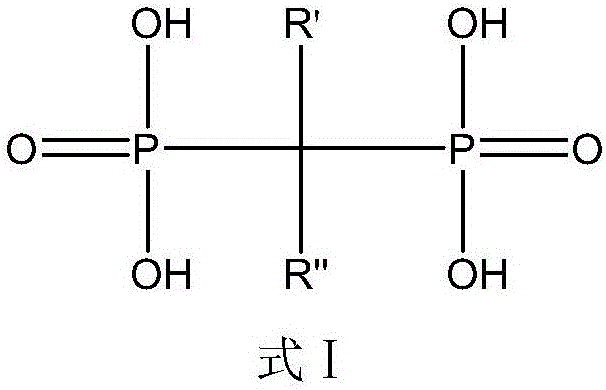
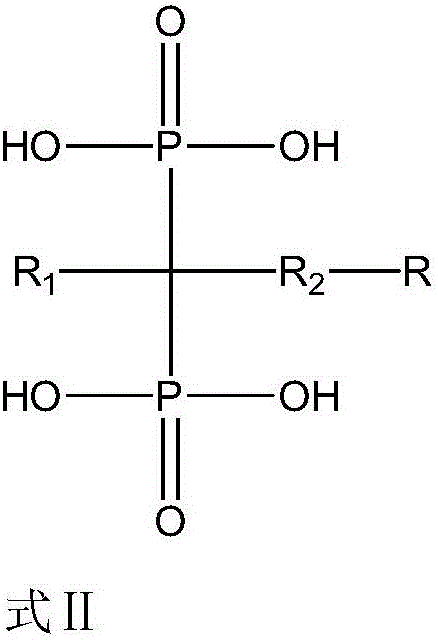
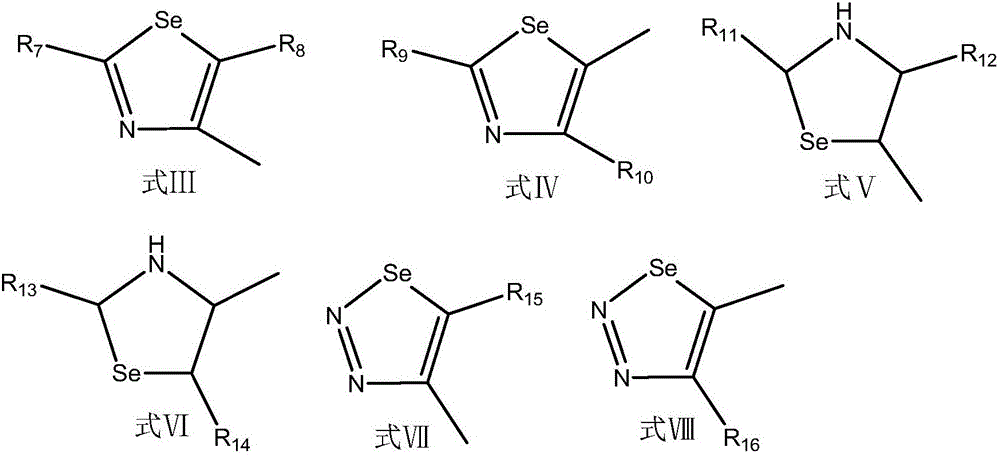
Diphosphoric acid compounds, and preparation method and application thereof
A compound, bisphosphonic acid technology, applied in the field of biomedicine, can solve problems such as little effect of osteoblasts and increased fractures in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1: Compound XC-1
[0084] In this example, the diphosphonic acid compound is: 1-hydroxy-2-(2-amino-1,3-selenazol-5-yl)-ethylidene-1,1-diphosphonic acid. The structural formula is as follows
[0085]
[0086] The specific preparation method of this compound is as follows:
[0087] Dissolve 1.43g (26.5mmol) sodium methoxide in 40mL of anhydrous methanol, add 1.14g (13.25mmol) γ-butyrolactone dropwise to the solution at room temperature, and react for 30min after the dropwise addition. Concentrate after the reaction, then add 20mL of water, adjust the pH to 3-4 with 2mol / L hydrochloric acid, then extract with ethyl acetate, collect the ether phase, and concentrate under reduced pressure to obtain the product 4-hydroxybutyric acid methyl ester.
[0088] Dissolve 1.18g (10mmol) of methyl 4-hydroxybutyrate in 30mL of dichloromethane, then add 0.13g (1.6mmol) of sodium acetate and 3.27g (15mmol) of pyridinium chlorochromate, and react at room temperature for 1h. ...
Embodiment 2
[0096] Example 2: Compound XC-2
[0097] The diphosphonic acid compound listed in this embodiment is: 1-hydroxy-2-(2-methyl-1,3-selenazol-5-yl)-ethylene-1,1-diphosphonic acid. The structural formula is as follows:
[0098]
[0099] The specific preparation method of this compound is as follows:
[0100] Dissolve 1.43g (26.5mmol) sodium methoxide in 40mL of anhydrous methanol, add 1.14g (13.25mmol) γ-butyrolactone dropwise to the solution at room temperature, and react for 30min after the dropwise addition. Concentrate after the reaction, then add 20mL of water, adjust the pH to 3-4 with 2mol / L hydrochloric acid, then extract with ethyl acetate, collect the ether phase, and concentrate under reduced pressure to obtain the product 4-hydroxybutyric acid methyl ester.
[0101] Dissolve 1.18g (10mmol) of methyl 4-hydroxybutyrate in 30mL of dichloromethane, then add 0.13g (1.6mmol) of sodium acetate and 3.27g (15mmol) of pyridinium chlorochromate, and react at room temperature ...
Embodiment 3
[0109] Embodiment 3: Compound XC-3
[0110] The diphosphonic acid compound listed in this embodiment is: 1-hydroxy-2-(2-imino-4-oxo-1,3-selazolidine-5-yl)-ethylene-1,1-diphosphonic acid . The structural formula is as follows:
[0111]
[0112] The specific preparation method of this compound is as follows:
[0113] Add 20.3g of L-aspartic acid, 4.3g of KBr, 44g of concentrated sulfuric acid, and 400mL of water into a 1L three-necked flask, install a thermometer, stir in an ice-salt bath to cool down to below 0°C, and slowly add 8.8 g NaNO 2 150mL aqueous solution, control the temperature in the reaction bottle below 0°C, brown gas will be produced, after the drop is completed, keep the temperature for 2h to obtain a light yellow reaction liquid. Extract with 4×100mL ethyl acetate, dry the organic phase for 2h, and distill the organic phase under reduced pressure to obtain bromosuccinic acid as a white solid product.
[0114] Add 7g of bromosuccinic acid, 150mL of ethan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com