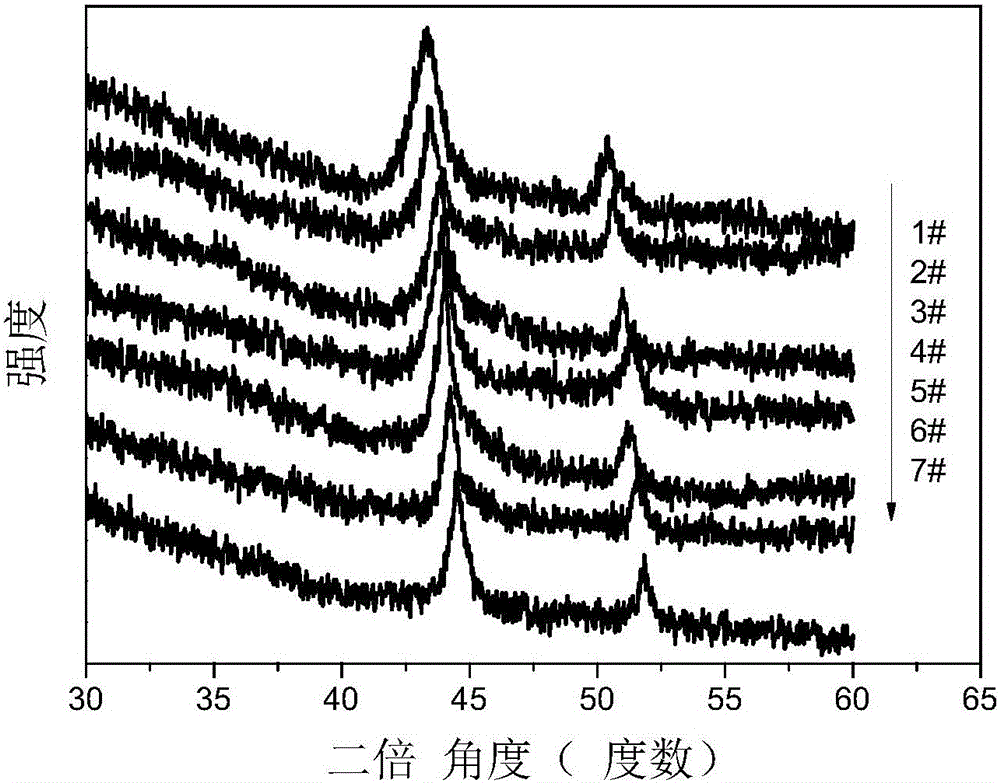
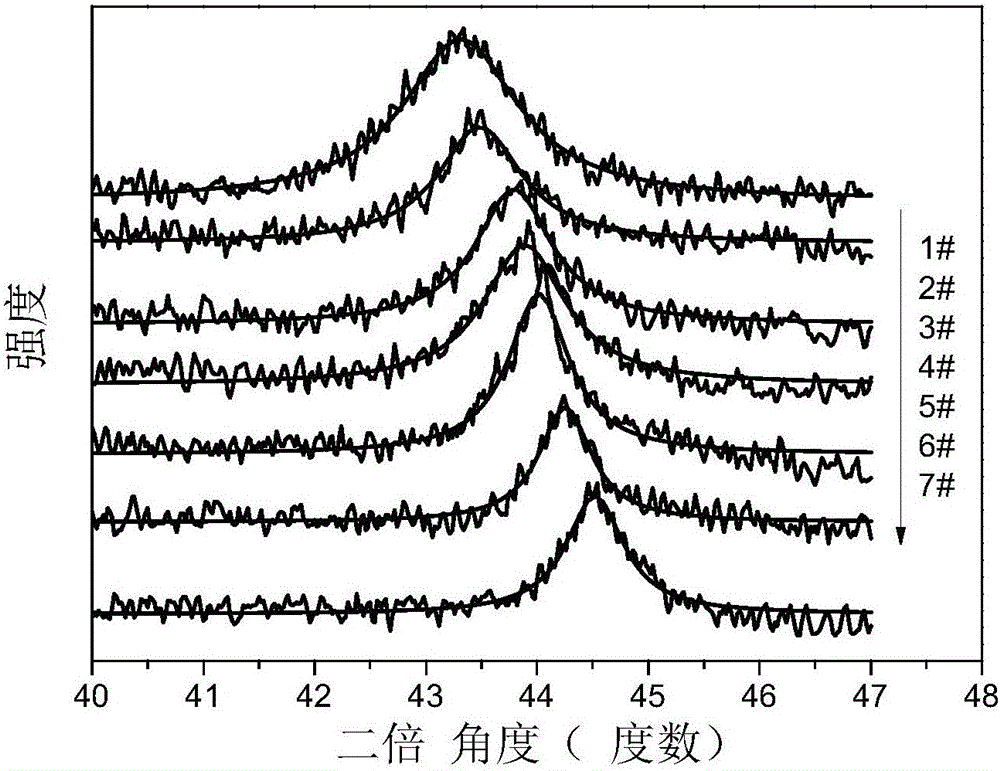
Application of copper-nickel nano alloys
A nano-alloy, copper-nickel technology, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problems of copper promoter performance not comparable to platinum, poor copper stability, failure, etc. Excellent photocatalytic hydrogen production from water splitting, excellent catalytic effect, and the effect of improving hydrogen production performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1) Polish the surface of the copper target with sandpaper, and at the same time remove part of the oxide on the surface of the copper target placed in the air. Then ultrasonic cleaning was performed in deionized water for 15 min, followed by ultrasonic cleaning in acetone for 15 min, and finally ultrasonic cleaning in absolute ethanol for 15 min. Take out the copper target, dry it in an oven, and soak it in isopropanol for use.
[0042] 2) Remove the copper target from the isopropanol, air-dry it naturally and place it in a glass reaction container, which is cylindrical, with a bottom diameter of 3.6 cm and a height of 15 cm. Inject 10 mL of isopropanol into the reaction vessel, so that the isopropanol immerses the copper target. The vertical distance between the isopropanol liquid level and the upper surface of the copper target is 0.8cm.
[0043]3) Adjust the output beam of the laser so that the laser beam is focused on the surface of the target after passing throug...
Embodiment 2
[0047] The difference from Example 1 is that this example changes the copper target into Cu 50 Ni 50 Target, 10mL reaction liquid isopropanol was replaced by 10mL isopropanol and water mixed solution (volume ratio: 1:0.7), and other preparation conditions remained the same as in Example 1. After the laser ablation reaction, dry to obtain the desired Cu 83 Ni 17 Nano alloy particles.
Embodiment 3
[0049] The difference from Example 1 is that this example changes the copper target into Cu 50 Ni 50 Target, 10mL of reaction liquid isopropanol was replaced by 10mL of isopropanol and water mixed solution (volume ratio 1:0.2), and other preparation conditions remained the same as in Example 1. After the laser ablation reaction, dry to obtain the desired Cu 63 Ni 37 Nano alloy particles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com