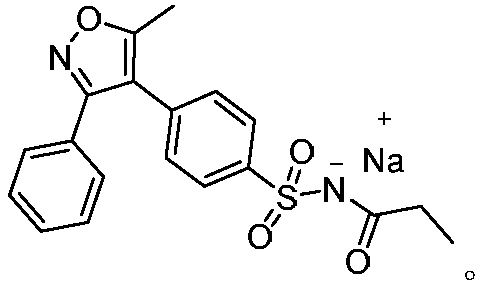
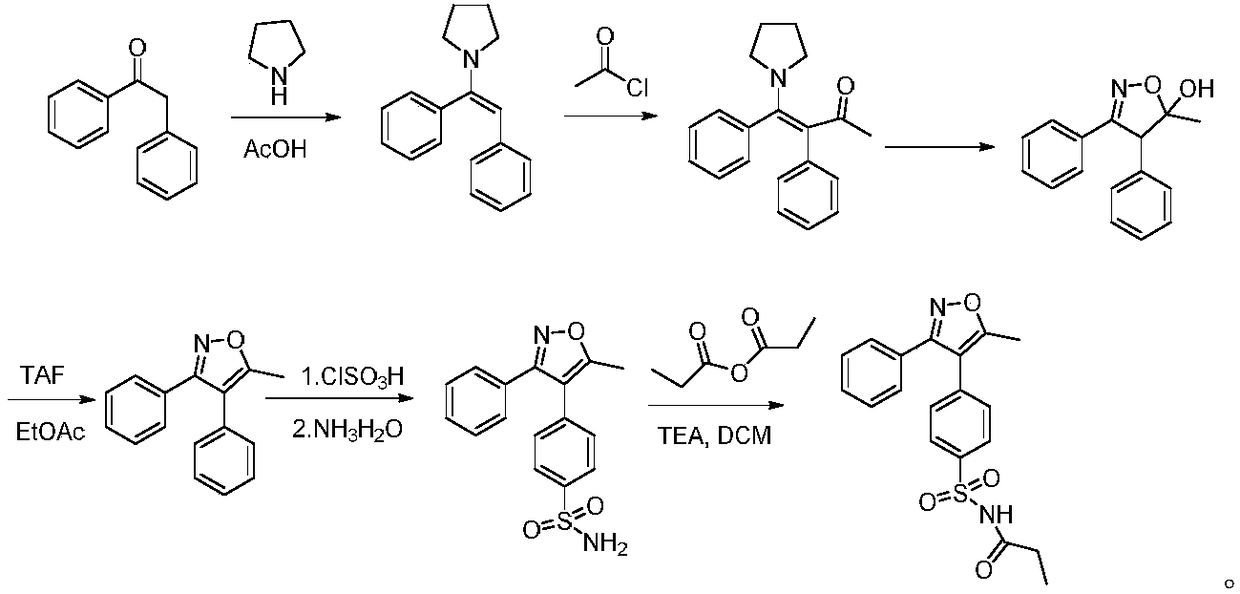
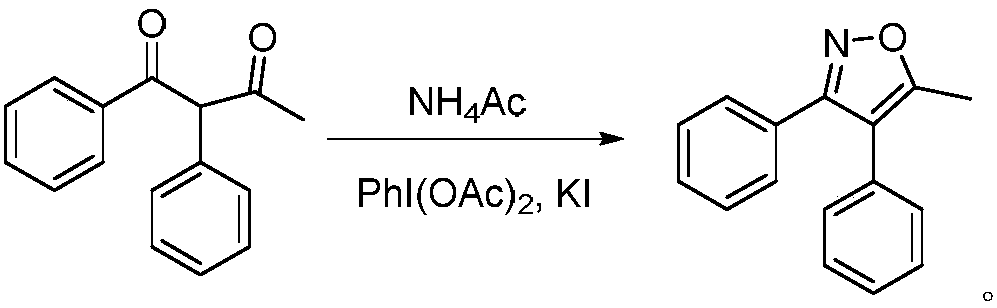A kind of method for preparing parecoxib intermediate
A technology of parecoxib and intermediates, applied in the field of drug synthesis, can solve the problems of complex reaction steps, high equipment requirements, fluoride environmental pollution, etc., and achieve the effect of increased reaction yield, simple reaction steps and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A method for preparing parecoxib intermediate, the method comprising: 1,2-diphenyl-2-acetyl ethyl ketone 29.1g (100mmol), ammonium acetate 23.1g (300mmol), iodobenzene diacetate 16.1 g (50mmol), potassium iodide 8.3g (50mmol) were added in the flask that 120ml dichloromethane was housed, 60 ℃ carried out contact reaction for 1 hour, after reaction finished, the organic phase was concentrated, washed with water, washed with saturated saline, anhydrous sodium sulfate Dry, then recrystallize from ethanol, and dry to obtain 21.1 g of 5-methyl-3,4-diphenylisoxazole, an intermediate of parecoxib, with a yield of 89.7% and a purity of 99.45%.
Embodiment 2
[0023] A method for preparing parecoxib intermediate, the method comprising: 1,2-diphenyl-2-acetyl ethyl ketone 29.1g (100mmol), ammonium acetate 38.5g (500mmol), iodobenzene diacetate 12.9 g (40mmol), potassium iodide 9.9g (60mmol) were added in the flask that 120ml dichloromethane was housed, 50 ℃ carried out contact reaction for 1 hour, after the reaction finished, the organic phase was concentrated, washed with water, washed with saturated saline, anhydrous sodium sulfate Dry, then recrystallize from ethanol, and dry to obtain 21.2 g of 5-methyl-3,4-diphenylisoxazole, an intermediate of parecoxib, with a yield of 90.1% and a purity of 99.39%.
Embodiment 3
[0025] A method for preparing parecoxib intermediate, the method comprising: 1,2-diphenyl-2-acetyl ethyl ketone 29.1g (100mmol), ammonium acetate 30.8g (400mmol), iodobenzene diacetate 12.9 g (40mmol) and potassium iodide 8.3g (50mmol) were added to a flask containing 120ml of dichloromethane, and the contact reaction was carried out at 55°C for 1 hour. After the reaction, the organic phase was concentrated, washed with water, washed with saturated brine, anhydrous sodium sulfate Dry, then recrystallize from ethanol, and dry to obtain 20.8 g of 5-methyl-3,4-diphenylisoxazole, an intermediate of parecoxib, with a yield of 88.6% and a purity of 99.41%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com