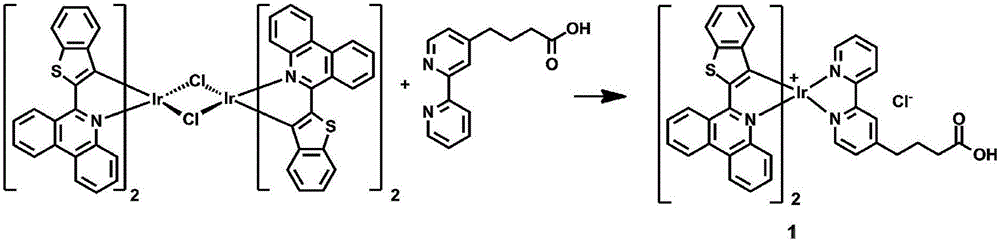
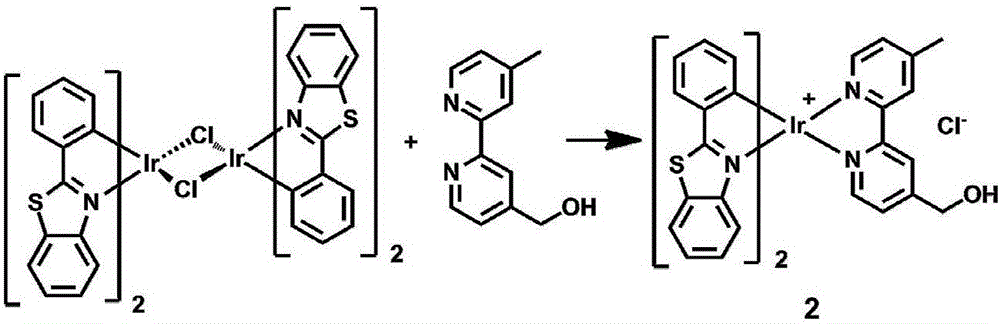
Dinuclear cyclic metal iridium complex as well as preparation method and application thereof
A technology of iridium complexes and ring metals, which is applied in the direction of indium organic compounds, platinum group organic compounds, chemical instruments and methods, etc., to achieve the effect of strong lipophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
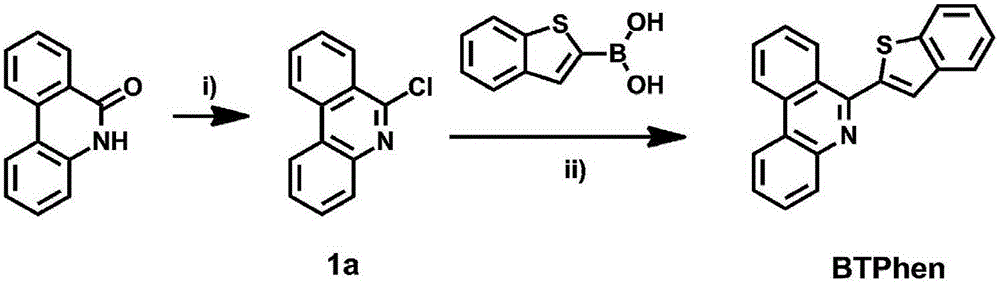
[0045] The present embodiment provides 6-chlorophenanthridine (structural formula is: ) synthetic route, such as figure 1 As shown, specifically:
[0046] 6(5H)-phenanthridone (5.11g, 26.2mmol) and phosphorus pentachloride (6.12g, 29.4mmol) were placed in a 250ml one-necked flask, 50ml of phosphorus oxychloride was added, and refluxed at 100°C for 2 hours under nitrogen protection ; Cool to room temperature, add 50ml of toluene to dilute the reaction solution, then spin off most of the excess phosphorus oxychloride, slowly pour the residual liquid into 2mol / L ammonia water, and extract the separated solid 3 times with 100ml of ethyl acetate; wash with water , dried over anhydrous magnesium sulfate and spin-dried to obtain 5.1 g of light yellow product. The resulting crude product was separated by silica gel column chromatography (EA:PE=2:3, v / v, the same below) to obtain 4.93 g of yellow flaky solid with a yield of 88.1%. figure 1 shown.
Embodiment 2
[0048] Present embodiment also provides 6-chlorophenanthridine (structural formula is: ) synthetic route, specifically:
[0049] Under nitrogen protection, 6(5H)-phenanthridinone (7.68g, 39.3mmol) was dissolved in 20ml SOCl 2 medium (0.5ml DMF as catalyst) at 85°C for 3 hours; after the reaction, spin dry excess SOCl 2 . Add dichloromethane to dissolve the residual solid, wash with saturated sodium bicarbonate solution three times, dry and spin dry, and put on a silica gel column; use DCM:PE=2:1 as the eluent to wash the column to separate the second component to obtain light yellow Flaky solid 5.49g, yield 65.3%. GC-MS: m / z (M + ) calcd 213.0, found 213.0. 1 H NMR (400MHz, CDC 3 )δ8.60(d,J=8.3Hz,1H),8.55-8.45(m,2H),8.09(dd,J=8.1,1.0Hz,1H),7.90(ddd,J=8.3,7.1,1.3Hz ,1H),7.79-7.65(m,3H).
Embodiment 3
[0051] The present embodiment provides 6-(2-benzo[b]thienyl)phenanthridine (BTPhen, the structural formula is: ) synthetic route, specifically:
[0052] Add 6-chlorophenanthridine (5.16g, 24.1mmol), 2-benzothiophene boronic acid (6.56g, 36.9mmol), Pd(PPh 3 ) 4 (1.47g, 1.27mmol) and anhydrous sodium carbonate (5.17g, 48.78mol), 24ml of water, 40ml of absolute ethanol and 80ml of toluene were added successively, and nitrogen replacement was performed three times after stirring evenly; then stirred and refluxed at 90°C for 12 hours; Cool to room temperature after the reaction, separate the toluene layer, extract the water phase with 100ml dichloromethane three times and merge into the toluene phase; spin dry and separate by silica gel column chromatography (DCM:PE=1:1) to obtain 6.84g white fine needles like crystals with a yield of 91.0%. GC-MS: m / z([M-H] + ) calcd 310.1, found 310.1. 1 H NMR (400MHz, CDCl 3 )δ8.70(d, J=8.3Hz, 1H), 8.65(d, J=8.3Hz, 1H), 8.59(d, J=7.8Hz, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com