3-hydrogenated pinicolic acid cyanide ethyl ester medicine and application thereof
A technology of cyanoethyl pine linate and ethyl acetate, which is used in drug combinations, steroids, antitumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
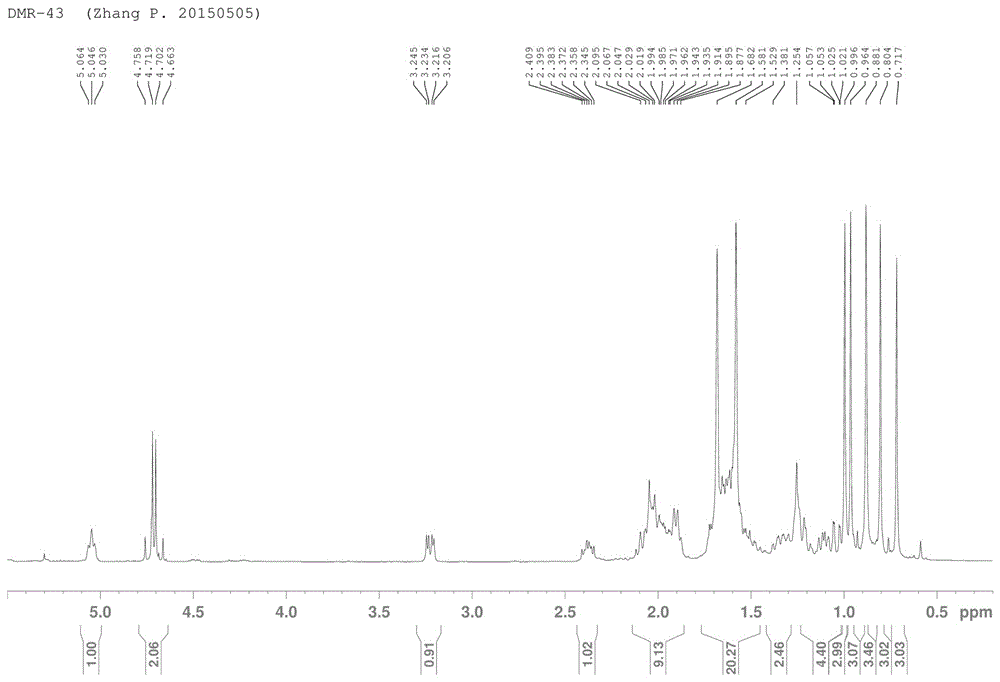
[0056] 3-Hydropine acid cyanoethyl ester, its system name is: (2R)-2-((3S,10S,13R,14R,17R)-3-hydroxy-4,4,10,13,14-pentamethyl Base-2,3,4,5,6,7,10,11,12,13,14,15,16,17-tetrahydro-1H-cyclopenta[a]phenanthrene-17-yl)-6- Methylhept-5-enoic acid cyanomethyl ester; its structural formula is:
[0057]
[0058] The synthetic method of 3-hydropinine acid cyanoethyl ester is:
[0059] 1) Add equimolar 3-hydropinoseic acid B, bromoacetonitrile, and potassium carbonate into the reaction flask, and add an appropriate amount of acetonitrile as a solvent, stir and react at 85°C for 2 hours, after the reaction is detected by TCL, spin to dry the solvent, and add water and ethyl acetate, separated and extracted three times, reclaimed ethyl acetate to obtain a solid;
[0060] 2) Load the solid by the solid method, and separate it by 200-300 mesh normal phase silica gel column chromatography, using petroleum ether: ethyl acetate = 15:1 (volume ratio) as the mobile phase to obtain a white so...
Embodiment 2
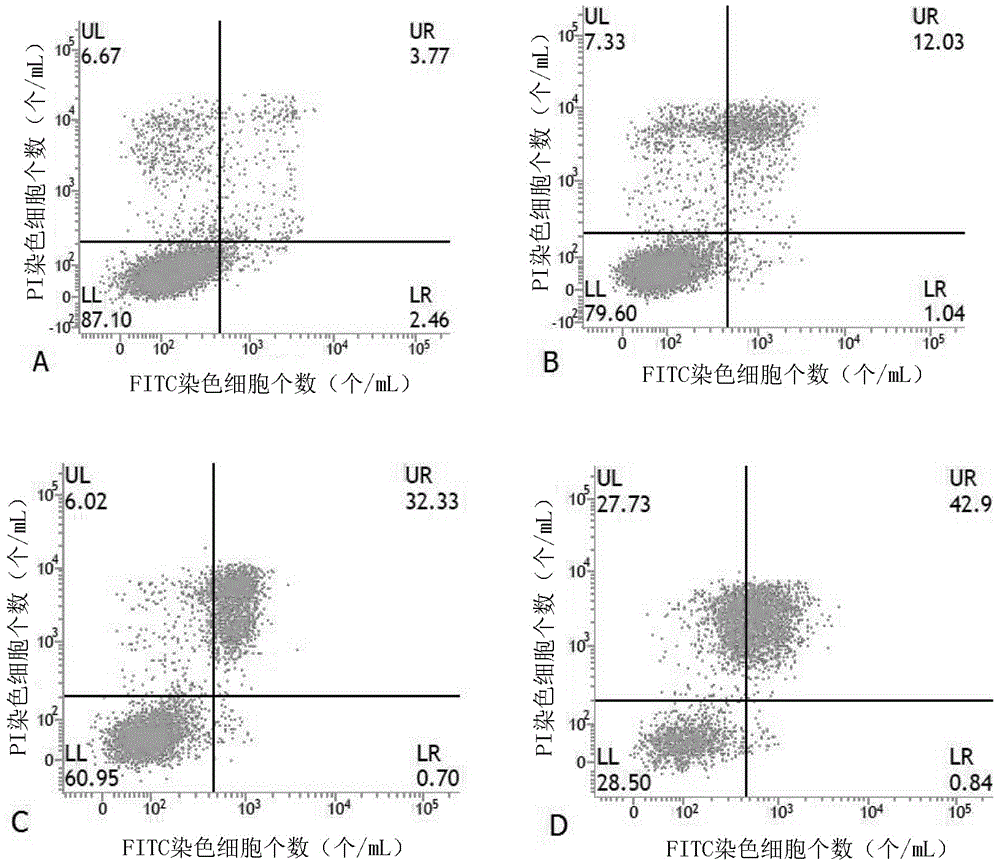
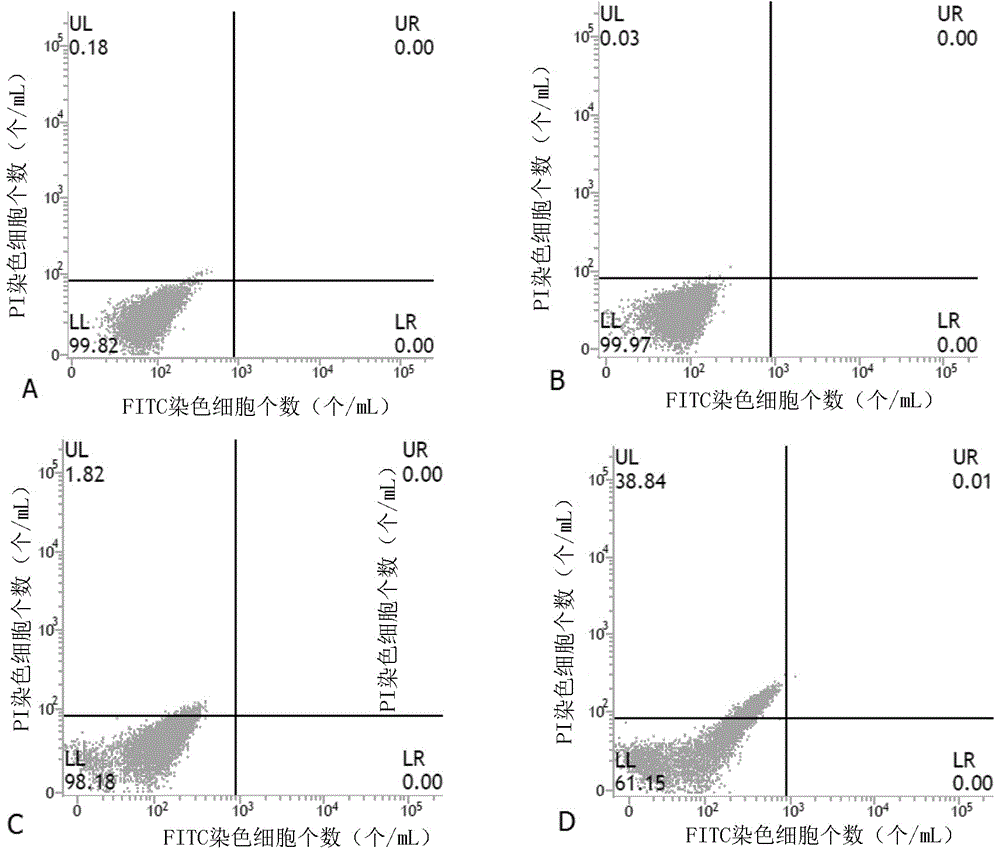
[0065] One, on the basis of embodiment 1, the obtained 3-hydropinine acid cyanoethyl ester is carried out concrete experiment, concrete method is as follows:
[0066] 1. Cell culture
[0067] Human gastric cancer cell line HGC-27, human liver cancer cell line HepG2, human lung cancer cell line A549, human breast cancer cell line MDA-MB-231, human prostate cancer cell line PC3, and human nasopharyngeal cancer cell line CNE-2, respectively Cultured in RPMI-1640 medium containing 10% fetal bovine serum, neuroblastoma SH-SY5Y, and human colon cancer HT-29 cell line were cultured in DMEM medium containing 10% fetal bovine serum, placed at 37°C and 5% CO 2 Open monolayer cell culture was carried out in a saturated humidity incubator. When the cell density is about 80% observed under an inverted microscope, subculture is carried out. When subculture, first discard the old culture medium, then wash twice with 5mL PBS, add 0.25% trypsin, and store at 37°C, 5% CO 2 Incubate in the i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com