Preparation method of nitride-boride fluorescent powder material
A technology for phosphors and raw materials, applied in the field of preparation of rare earth or Mn2+ ion-activated nitrogen boride phosphor materials, can solve the problems of high price, harsh environment, unsuitable for industrial large-scale production, etc., and achieves low cost and short production cycle. , the effect of cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1, LiSr 4 (BN 2 ) 3 : Preparation of Cel mol%
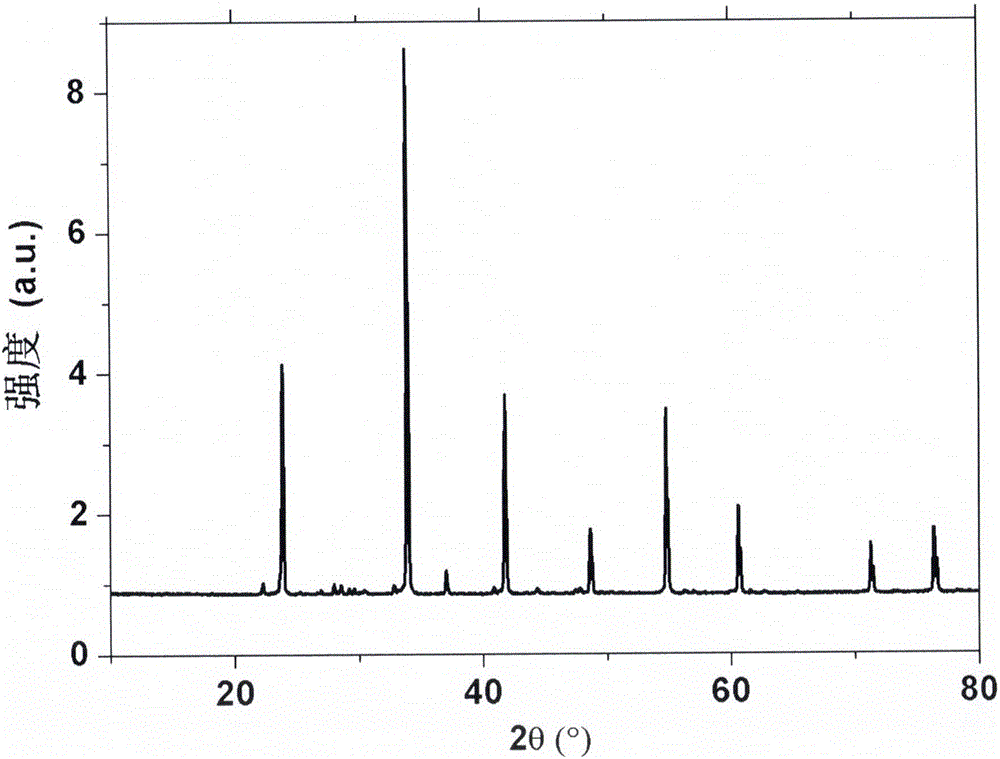
[0048] Weigh 0.185g Li according to the stoichiometric ratio 2 CO 3 , 4.190g Sr(NO 3 ) 2 , 0.927g HBO 3 , 0.035g CeO 2 . Put the weighed raw materials into an agate grinder, add acetone and grind continuously until the acetone is completely volatilized, and all the raw materials are mixed uniformly. The mixture was then placed in a corundum crucible and sintered at 650°C for 3 hours under charcoal thermal conditions. The obtained sintered product was reground uniformly, then put into corundum crucible, sintered at 800℃ for 3 hours under the condition of carbon heat, and obtained LiSr after grinding 4 (BN 2 ) 3 : Celmol% phosphor. XRD powder diffraction indicated that pure LiSr was obtained 4 (BN 2 ) 3 structure( figure 1 ).
Embodiment 2
[0049] Example 2, LiSr 4 (BN 2 ) 3 : Preparation of Ce2mol%
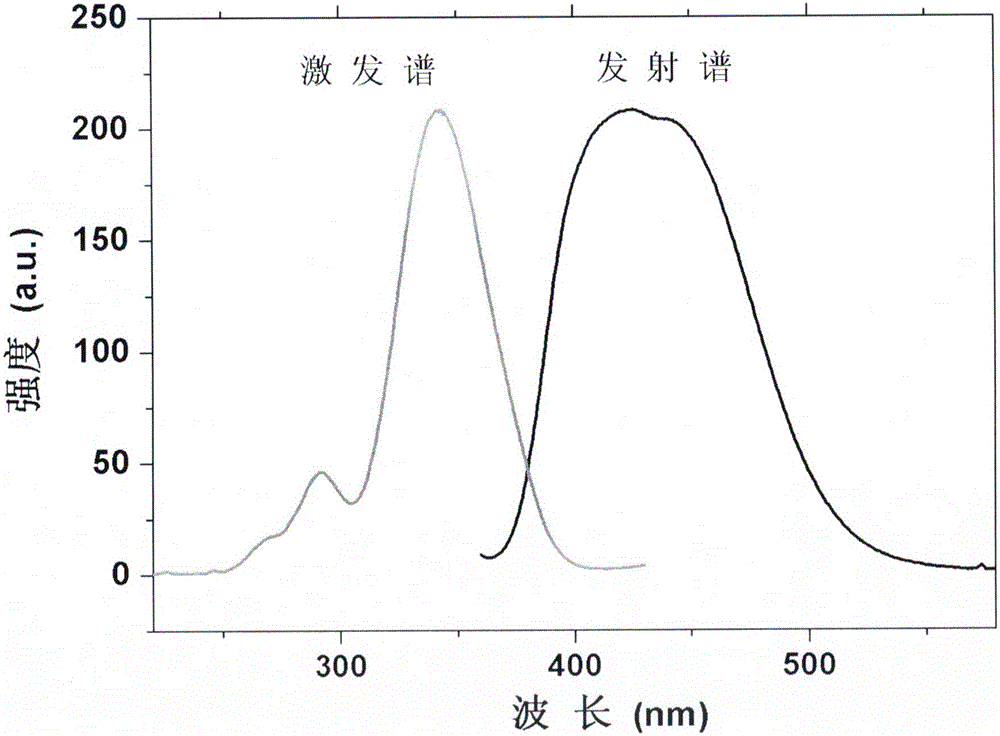
[0050] Weigh 0.185g Li according to the stoichiometric ratio 2 CO 3 , 4.146g Sr (NO 3 ) 2 , 0.927g HBO 3 , 0.069g CeO 2 . Put the weighed raw materials into an agate grinder, add acetone and grind continuously until the acetone is completely volatilized, and all the raw materials are mixed uniformly. The mixture was then placed in a corundum crucible and sintered at 650°C for 3 hours under charcoal thermal conditions. The obtained sintered product was reground uniformly, then put into corundum crucible, sintered at 800℃ for 3 hours under the condition of carbon heat, and obtained LiSr after grinding 4 (BN 2 ) 3 : Ce2mol% phosphor. Use a fluorescence spectrometer to measure the excitation and emission spectra of phosphors, such as figure 2 shown. Under the excitation of near-ultraviolet light, the phosphors exhibit strong blue emission.
Embodiment 3
[0051] Example 3, LiSr 4 (BN 2 ) 3 : Preparation of Ce 0.5mol%, Tb2mol%
[0052] Weigh 0.185g Li according to the stoichiometric ratio 2 CO 3 , 4.169g Sr(NO 3 ) 2 , 0.927g HBO 3 , 0.017g CeO 2 , 0.083g Tb (NO 3 ) 3 4(H 2 O). Put the weighed raw materials into an agate grinder, add acetone and grind continuously until the acetone is completely volatilized, and all the raw materials are mixed uniformly. The mixture was then placed in a corundum crucible and sintered at 650°C for 3 hours under charcoal thermal conditions. The obtained sintered product was reground uniformly, then put into corundum crucible, sintered at 800℃ for 3 hours under the condition of carbon heat, and obtained LiSr after grinding 4 (BN 2 ) 3 : Ce2mol% phosphor. Use a fluorescence spectrometer to measure the excitation and emission spectra of phosphors, such as figure 2 shown. Under the excitation of near-ultraviolet light, the phosphors exhibit strong blue emission.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com