Method for continuously preparing low-cost battery grade ferric phosphate by utilizing iron filings
A ferric orthophosphate, battery-grade technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of increasing equipment investment and operating costs, increasing the burden of washing and filtration, and large consumption of deionized water, etc. Achieve the effect of less washing water consumption, easy selection of separation device and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Phosphoric acid solution preparation
[0044] Weigh 100kg of phosphoric acid and add 325kg of deionized water and stir evenly to prepare a phosphoric acid solution with a concentration of 20% for later use; weigh 100kg of hydrogen peroxide with a concentration of 30% and add 400kg of deionized water and stir evenly to prepare a hydrogen peroxide solution with a concentration of 6% for later use , drive the prepared phosphoric acid solution and hydrogen peroxide solution into the high level tank with an explosion-proof pump;
[0045] (2) Dissolution-reaction
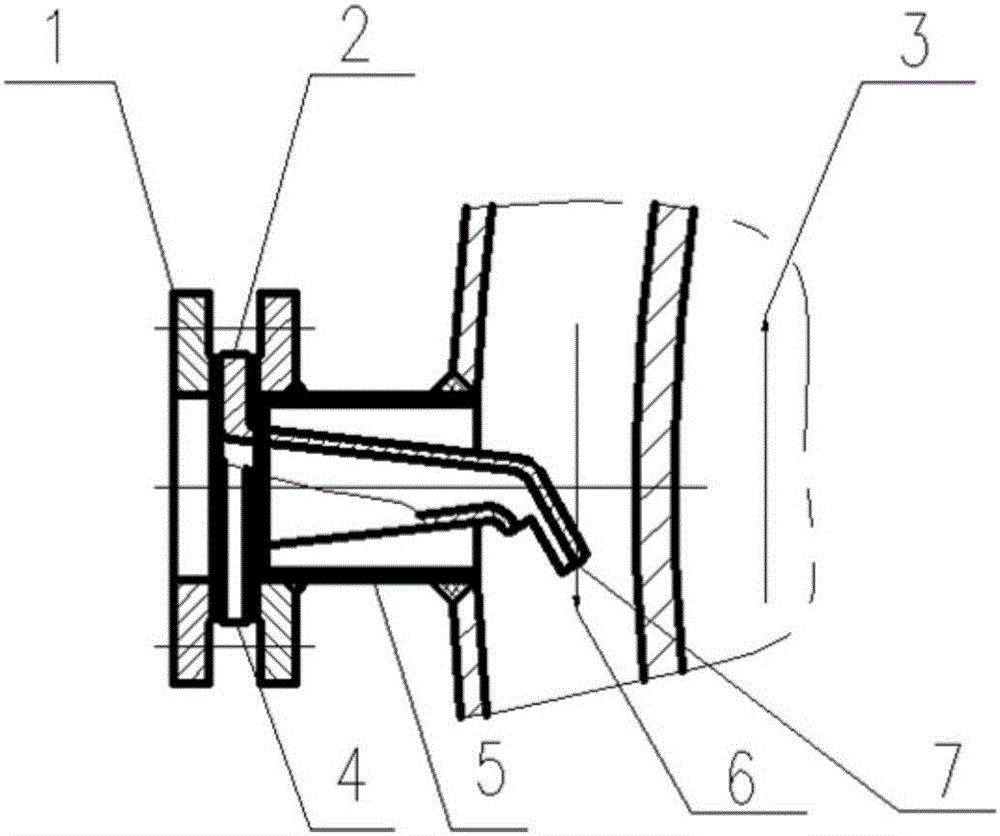
[0046]Weigh 42.5kg of iron red into the reaction kettle, and add 85kg of deionized water to stir vigorously. At the beginning, raise the temperature to the set temperature of 95°C. When the temperature rises to the set temperature, use the anti-corrosion pressure pump to adjust the step (1) 127.5kg of a good phosphoric acid solution is injected into the reactor feed port 4 and the air pressure nozzle 7 install...
Embodiment 2
[0054] (1) Solution preparation
[0055] Weigh 120kg of phosphoric acid and add 390kg of deionized water and stir evenly to prepare a phosphoric acid solution with a concentration of 85% for use. Weigh 100kg of hydrogen peroxide with a concentration of 28% and add 400kg of deionized water to mix evenly, and prepare a hydrogen peroxide solution with a concentration of 6% for use , drive the prepared phosphoric acid solution and hydrogen peroxide solution into the high level tank with an explosion-proof pump;
[0056] 2) Dissolution-reaction
[0057] Weigh 40kg of iron powder and pour it into the reaction kettle, add 350kg of deionized water and stir. At the beginning, the temperature rises to the set temperature of 45°C; 400kg is driven into the reactor feed port 4, and the pneumatic nozzle 7 installed at the 4 places starts to feed, and keeps stirring vigorously. The feeding time takes 2 hours to finish adding the phosphoric acid solution, and keeps stirring vigorously. The ...
Embodiment 3
[0066] (1) Solution preparation
[0067] Weigh 100kg of phosphoric acid and add 70kg of deionized water to stir evenly to make a 50% phosphoric acid solution for use. Weigh 100kg of hydrogen peroxide with a concentration of 30% and add 400kg of deionized water to stir evenly to prepare a 6% hydrogen peroxide solution for use. The good phosphoric acid solution and hydrogen peroxide solution are pumped into the high-level tank with an explosion-proof pump;
[0068] (2) Dissolution-reaction
[0069] Weigh 40 kilograms of cut iron scraps and place them in the reactor, add 200 kilograms of deionized water and stir slowly, and start the temperature rise to the set temperature of 90°C; after the temperature rises to the set temperature, use the anti-corrosion pressure pump to turn the step (1 ) 280 kg of phosphoric acid solution prepared into the reaction kettle feed port 4 installed pneumatic nozzle 7 began to feed, and slowly stirred, after adding phosphoric acid solution in 2 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com