Metal organic frame fluorescent probe and application thereof in hydrogen sulfide detection
A metal-organic framework, fluorescent probe technology, applied in 3/13 groups of organic compounds without C-metal bonds, organic chemistry, fluorescence/phosphorescence, etc., can solve the problem of poor selectivity, complex working environment of small molecule fluorescent probes, The synthesis is cumbersome and complicated, and the effects of high selectivity, high yield and simple synthesis route are achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
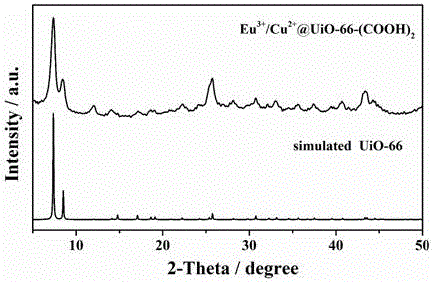
[0027] (1) Add ZrCl to the round bottom flask 4 (2.43 g, 10.4 mmol), pyromellitic acid (2.54 g, 10 mmol), deionized water (60 mL) and glacial acetic acid (40 mL), stirred at room temperature for 30 min, and placed in an oil bath at 100 °C Condensation and reflux reaction for 24 h;
[0028] After cooling to room temperature, centrifuge, disperse the precipitate in deionized water, repeat centrifugal washing 3 times, after centrifugation, the obtained precipitate is UiO-66-(COOH) containing uncoordinated carboxyl group 2 ;
[0029] (2) Take the UiO-66-(COOH) synthesized above 2 (0.1 g) was uniformly dispersed in deionized water (10 mL), and Eu(NO 3 ) 3 ·6H 2 O (0.446 g, 1 mmol) and Cu(NO 3 ) 2 2.5H 2 O (0.465 g, 1 mmol), condensed and refluxed at 60°C for 24 h; cooled to room temperature and centrifuged, dispersed the precipitate in deionized water, centrifuged and washed 3 times repeatedly, after centrifuged, exchanged with acetone for 3 days, and replaced every day ...
Embodiment 2
[0033] (1) Add Zr(NO 3 ) 4 ·5H 2 O (4.46 g, 10.4 mmol), pyromellitic acid (2.10 g, 10 mmol), deionized water (60 mL) and glacial acetic acid (40 mL), stirred at room temperature for 30 min, and placed in an oil bath at 100 °C Condensation and reflux reaction in middle temperature for 24 h; after cooling to room temperature and centrifugation, the precipitate was dispersed in deionized water, and centrifuged and washed 3 times repeatedly. After centrifugation, the obtained precipitate was UiO-66-(COOH) containing uncoordinated carboxyl groups. 2 ;
[0034] (2) Take the UiO-66-(COOH) synthesized above 2 (0.1 g) was uniformly dispersed in deionized water (10 mL), and EuCl 3 ·6H 2 O (0.366 g, 1 mmol) and Cu(NO 3 ) 2 2.5H 2 O (0.465 g, 1 mmol), stirred and condensed at 90°C for reflux for 12 hours; cooled to room temperature and centrifuged, dispersed the precipitate in deionized water, repeated centrifuged and washed 3 times, after centrifuged, exchanged with acetone for ...
Embodiment 3
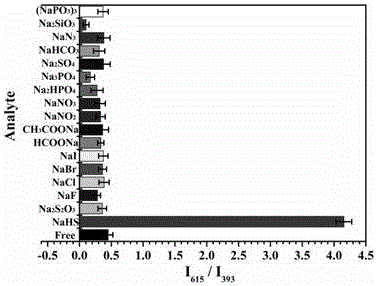
[0036] Example 3 Selectivity, response time and sensitivity of metal-organic framework fluorescent probes for hydrogen sulfide detection
[0037] (1) Take 1 mL of 5 mM containing F - , Cl - , Br - , I - , HS - , HCOO - , CH 3 COO - , NO 2 - , SiO 3 2- , NO 3 - , HPO 4 2- , PO 4 3- , P 3 o 9 3- , S 2 o 3 2- , SO 4 2- , HCO 3 - , and N 3 - The sodium salt solution of the anion is placed in a cuvette, and 1 mg of fluorescent probe Eu is added to each cuvette 3+ / Cu 2+ @UiO-66-(COOH) 2 , shake well, and measure its fluorescence emission spectrum at an excitation wavelength of 305 nm after 30 seconds (such as image 3 shown), the results show that adding these anions: F - , Cl - , Br - , I - , HCOO - , CH 3 COO - , NO 2 - , SiO 3 2- , NO 3 - , HPO 4 2- , PO 4 3- , P 3 o 9 3- , S 2 o 3 2- , SO 4 2- , HCO 3 - , and N 3 - The sodium salt solution of the fluorescent probe Eu 3+ / Cu 2+ @UiO-66-(COOH) 2 The fluorescence inten...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com