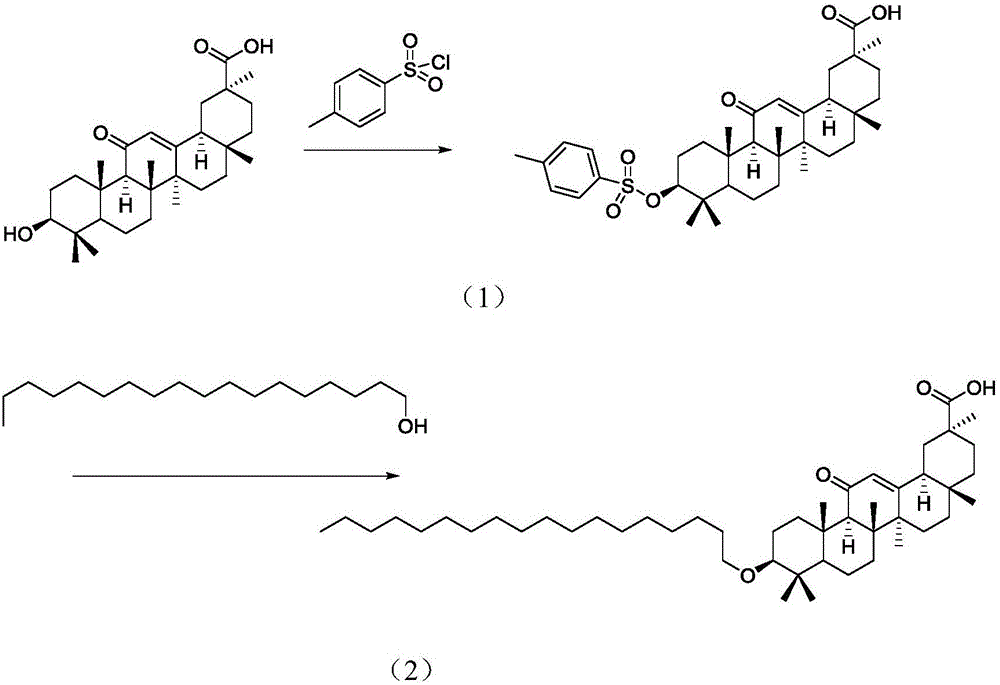
Preparation method and intermediate of stearyl glycyrrhetinate
A technology of octadecyl glycyrrhetinate and glycyrrhetinic acid, applied in the directions of steroids, organic chemistry, etc., can solve the problems of high market price, the output can not meet the market demand, etc., and achieves low production cost, easy promotion, and stability. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
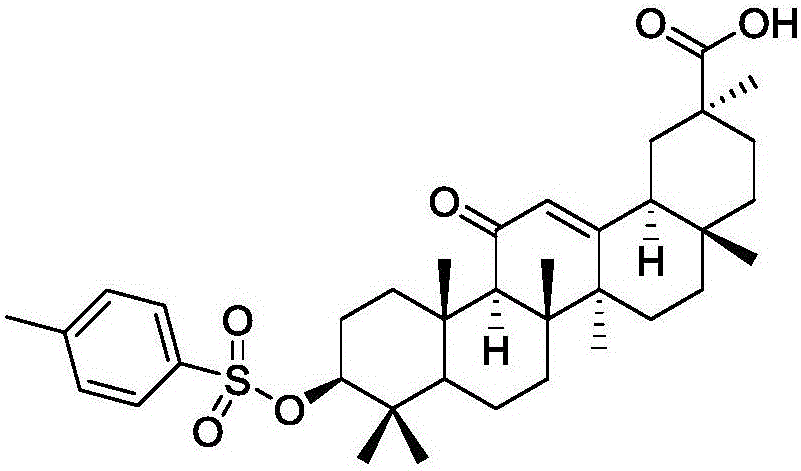
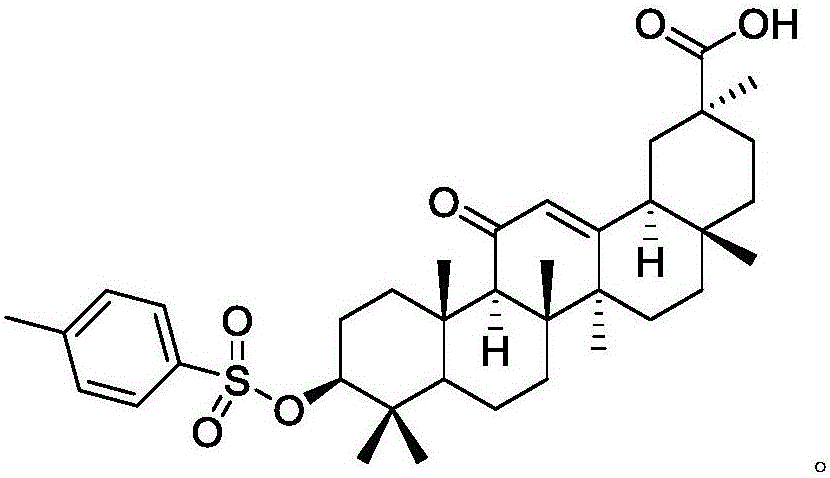
[0030] Add 100 ml of ethyl acetate and 50 ml of triethylamine to 1 mol of glycyrrhetinic acid and 1 mol of p-toluenesulfonyl chloride, stir at room temperature (25°C) for 2 hours, a white solid precipitates, and filter. Add 1 mol of the above white solid, 1 mol of stearyl alcohol, and 1.5 mol of potassium carbonate to 200 ml of DMF and react at 70°C for 5 hours, add water, extract with ethyl acetate, and dry the organic phase to obtain 685.9 g of the product with a yield of 95%.
[0031] Detection method: Shimadzu LC-20AT liquid chromatograph, with InertSustain C18 column (4.6×150mm×5μm) as chromatographic column, acetonitrile:1mol / L phosphoric acid aqueous solution (85:15) as mobile phase, detection wavelength 254nm, The flow rate is 1.0ml / min. HPLC purity 98.3%.
Embodiment 2
[0033] Add 150 ml of ethyl acetate and 50 ml of triethylamine to 1 mol of glycyrrhetinic acid and 1 mol of p-toluenesulfonyl chloride, stir at room temperature for 2 hours, a white solid precipitates, and filter. 1 mol of white solid, 1 mol of stearyl alcohol, and 2 mol of potassium carbonate were added to 200 ml of DMF to react at 70° C. for 5 hours, water was added, extracted with ethyl acetate, and the organic phase was dried to obtain 649.8 g of the product with a yield of 90%.
[0034] Detection method: Shimadzu LC-20AT liquid chromatograph, with InertSustain C18 column (4.6×150mm×5μm) as chromatographic column, acetonitrile:1mol / L phosphoric acid aqueous solution (85:15) as mobile phase, detection wavelength 254nm, The flow rate is 1.0ml / min, and the HPLC purity is 92.9%.
Embodiment 3
[0036] Add 100 ml of ethyl acetate and 50 ml of triethylamine to 1 mol of glycyrrhetinic acid and 1.5 mol of p-toluenesulfonyl chloride, stir at room temperature for 2 hours, a white solid precipitates, and filter. 1 mol of the above white solid, 1 mol of stearyl alcohol, and 1.5 mol of potassium carbonate were added to 250 ml of DMF to react at 70° C. for 5 hours, add water, extract with ethyl acetate, and dry to obtain 664.24 g of the product with a yield of 92%.
[0037] Detection method: Shimadzu LC-20AT liquid chromatograph, with InertSustain C18 column (4.6×150mm×5μm) as chromatographic column, acetonitrile:1mol / L phosphoric acid aqueous solution (85:15) as mobile phase, detection wavelength 254nm, The flow rate is 1.0ml / min, and the HPLC purity is 93.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com