A kind of multi-functional photocurable resin for color photoresist and preparation method thereof
A light-curing resin and multi-functionality technology, which is applied in the field of multi-functionality light-curing resin and its preparation, can solve the problems of increased coating volume shrinkage, decreased water and weather resistance, and environmental pollution, achieving small volume shrinkage and good Excellent hardness, aging resistance and chemical corrosion resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
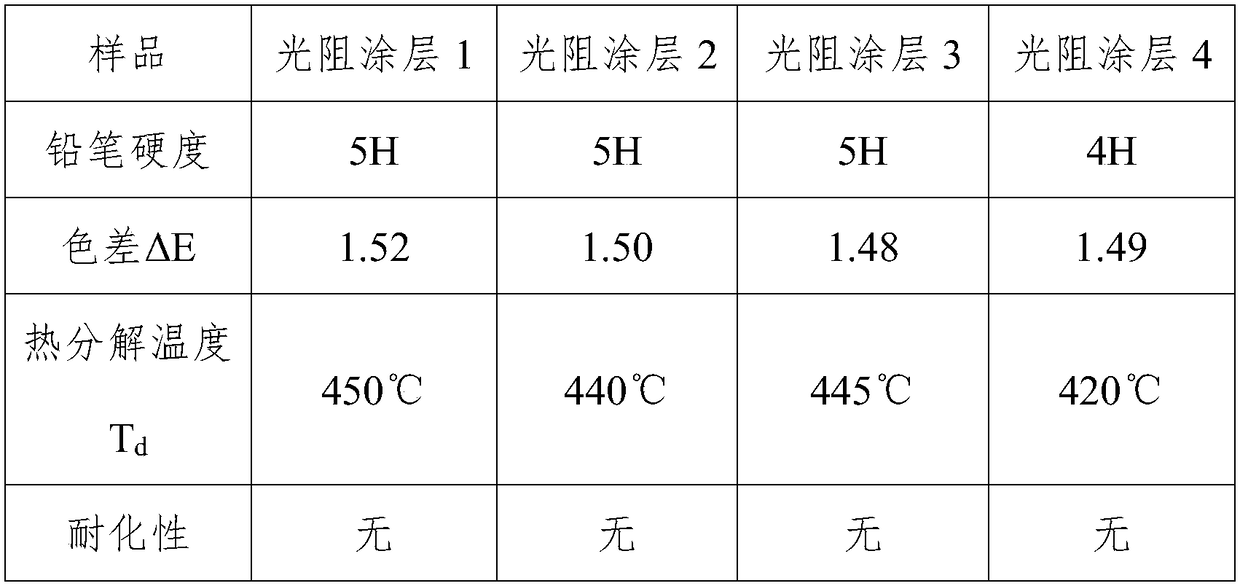
Examples
Embodiment 1
[0016] (1) Add gallic acid, pyromellitic anhydride, and hydrochloric acid accounting for 5wt% of the reactant (concentration 37wt%, hereinafter the same ), with acetone as the reaction solvent, heated to 60°C to condense and reflux. Samples were taken every 30 minutes to analyze the esterification rate of pyromellitic anhydride by infrared. To be anhydride at 1847cm -1 The infrared peak at 3600cm decreases to no longer changes -1 The hydroxyl peak of α was also reduced to no longer changed, indicating a complete reaction. The solvent was then distilled off under reduced pressure to obtain a solid powder product.
[0017] (2) Add 5g of the product of step (1) and 0.005g of hydrochloric acid into a 250mL four-neck flask equipped with a water separator, heat to melt and start stirring, and when the temperature of the system rises to 200°C, add 0.5g of methyl Hydroxyethyl acrylate (containing 0.05g of hydroquinone), the rate of addition is based on the fact that the temperatur...
Embodiment 2
[0019] (1) Add gallic acid, pyromellitic anhydride, and hydrochloric acid accounting for 0.1wt% of the reactant in a 250mL four-necked flask with a molar ratio of 1:5, using acetone as the reaction solvent , heated to 60°C and condensed to reflux. Samples were taken every 30 minutes to analyze the esterification rate of pyromellitic anhydride by infrared. To be anhydride at 1847cm -1 The infrared peak at 3600cm decreases to no longer changes -1 The hydroxyl peak of the product was also reduced to no longer changing, indicating that after sufficient reaction, the solvent acetone was distilled off under reduced pressure to obtain a solid powder product.
[0020] (2) Add 5g of the product of step (1) and 0.005g of hydrochloric acid into a 250mL four-neck flask equipped with a water separator, heat to melt and start stirring, and when the temperature of the system rises to 200°C, add 0.5g of methyl For hydroxyethyl acrylate (containing 0.05g of hydroquinone), the rate of additi...
Embodiment 3
[0022] (1) add gallic acid, pyromellitic anhydride, and account for reactant 5wt% sulfuric acid in a 250mL four-neck flask with a molar ratio of 1:1, with acetone as the reaction solvent, Heated to 60°C and condensed to reflux. Samples were taken every 30 minutes to analyze the esterification rate of pyromellitic anhydride by infrared. To be anhydride at 1847cm -1 The infrared peak at 3600cm decreases to no longer changes -1 The hydroxyl peak of the product was also reduced to no longer changing, indicating that after sufficient reaction, the solvent acetone was distilled off under reduced pressure to obtain a solid powder product.
[0023] (2) Add 5g of the product of step (1) and 0.25g of sulfuric acid into a 250mL four-neck flask equipped with a water separator, heat to melt and start stirring. When the temperature of the system rises to 200°C, add 0.5g of methyl For hydroxyethyl acrylate (containing 0.05g of hydroquinone), the rate of addition is based on the fact that ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com