Preparation method for titanium dioxide nanotube array decorated with cobalt and nickel double-layer hydroxide and application of photoelectron-chemistry hydrolysis hydrogen production
A technology of double-layer hydroxide and nanotube arrays, which is applied in the direction of nanotechnology, nanotechnology, nanotechnology, etc. for materials and surface science, can solve the problems of difficult and efficient use of sunlight and low photoelectric catalytic activity, and achieve Easy to operate, easy to industrialize, and improve the effect of photoelectrochemical activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
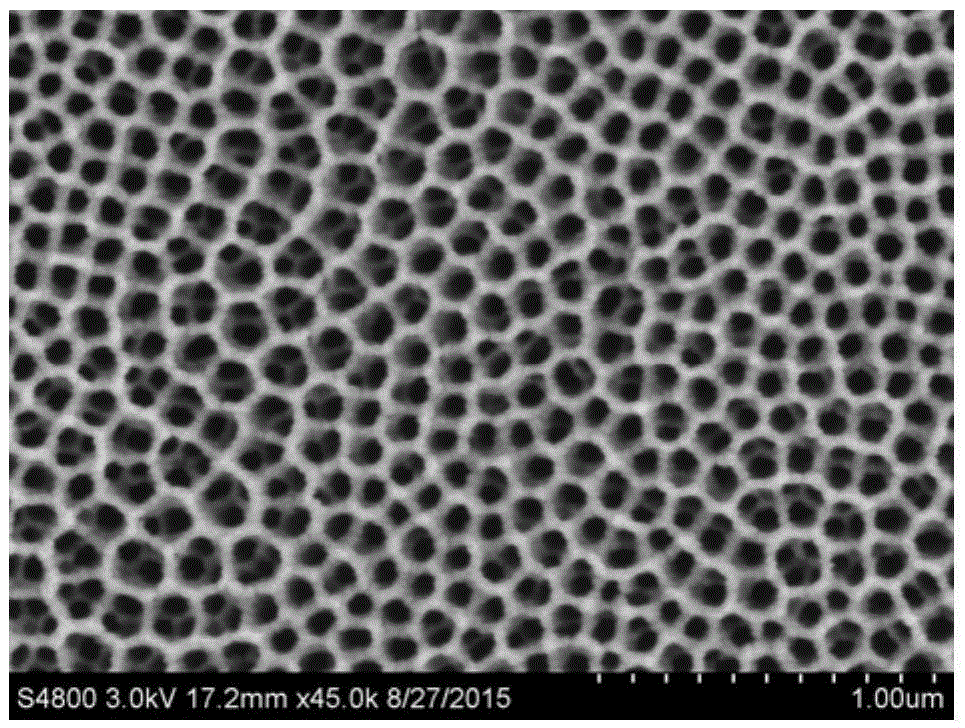
[0049] Polish a smooth titanium sheet (1×3cm 2 ) surface cleaned and air dried. Dissolve 0.5g of ammonium fluoride in 100mL of hexanediol aqueous solution, stir evenly, immerse one end of the cleaned titanium piece in the above solution, and clamp the other end with the electrode clamp of the potentiostat, and control the voltage at 50V. 2h. The samples were taken out, washed alternately with ethanol and deionized water, placed in a vacuum oven at 60°C, and dried for 5 hours. Put it into a muffle furnace and heat it at 600°C for 2h. Next, prepare CoCl at a concentration of 5 mM 2 ·6H 2 O, Ni(NO 3 ) 2 ·6H 2 O mixed solution, 50mL was placed in a beaker, and placed in a three-electrode system for electrodeposition at a constant potential of -1V for 5s. The samples were taken out and washed alternately with ethanol and deionized water, and stored in a vacuum oven. figure 1 For the prepared TiO 2 SEM image of nanotube arrays. shows that at a small magnification, the TiO...
Embodiment 2
[0051] Polish a smooth titanium sheet (1×3cm 2 ) surface cleaned and air dried. Dissolve 0.5g of ammonium fluoride in 100mL of hexanediol aqueous solution, stir evenly, immerse one end of the cleaned titanium piece in the above solution, and clamp the other end with the electrode clamp of the potentiostat, and control the voltage at 50V. 2h. The samples were taken out, washed alternately with ethanol and deionized water, placed in a vacuum oven at 60°C, and dried for 5 hours. Put it into a muffle furnace and heat it at 600°C for 2h. Next, prepare CoCl at a concentration of 5 mM 2 ·6H 2 O, Ni(NO 3 ) 2 ·6H 2 O mixed solution, 50mL was placed in a beaker, and placed in a three-electrode system for electrodeposition at a constant potential of -1V for 20s. The samples were taken out and washed alternately with ethanol and deionized water, and stored in a vacuum oven.
Embodiment 3
[0053] Polish a smooth titanium sheet (1×3cm 2 ) surface cleaned and air dried. Dissolve 0.5g of ammonium fluoride in 100mL of hexanediol aqueous solution, stir evenly, immerse one end of the cleaned titanium piece in the above solution, and clamp the other end with the electrode clamp of the potentiostat, and control the voltage at 50V. 2h. The samples were taken out, washed alternately with ethanol and deionized water, placed in a vacuum oven at 60°C, and dried for 5 hours. Put it into a muffle furnace and heat it at 600°C for 2h. Next, prepare CoCl at a concentration of 5 mM 2 ·6H 2 O, Ni(NO 3 ) 2 ·6H 2 O mixed solution, 50mL was placed in a beaker, and placed in a three-electrode system for electrodeposition at a constant potential of -1V for 30s. The samples were taken out and washed alternately with ethanol and deionized water, and stored in a vacuum oven.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com