Application of xylaria nigriper powder to preparation of medicines or healthcare food for preventing and treating osteoporosis and osteonecrosis
A technology for osteoporosis and health food, applied in the field of traditional Chinese medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
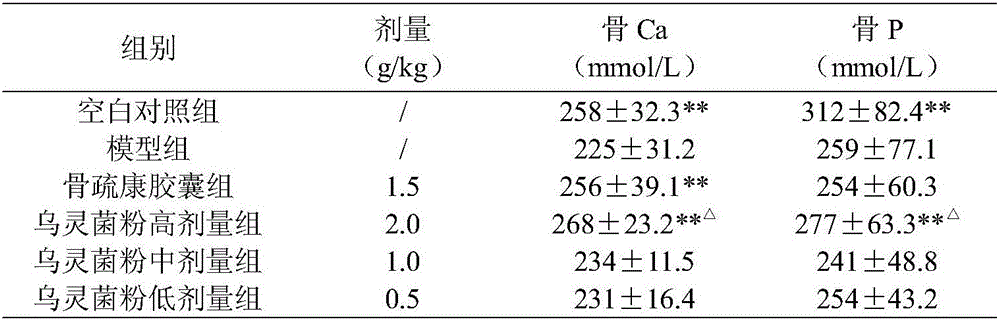
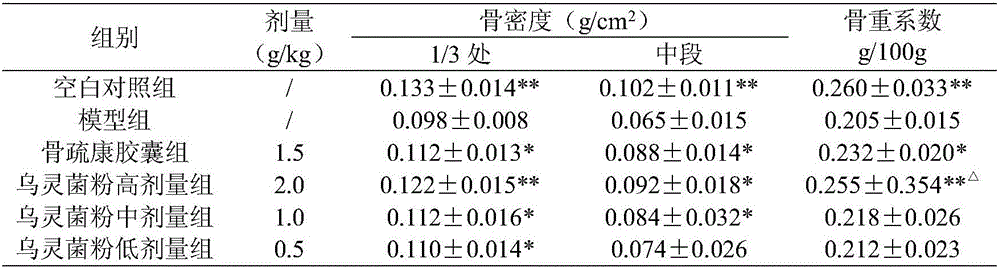
[0025] Example 1 Pharmacodynamic Study on Osteoporosis Treated by Wulingjun Powder
[0026] 1 material
[0027] 1.1 Experimental animals
[0028] Wistar male rats, weighing 180-220 g.
[0029] 1.2 Drugs and reagents
[0030] Wuling mushroom powder: provided by Hangzhou Zhongzhi Kanggu Biotechnology Co., Ltd., batch number JC16011401. Positive drug: Gushukang Capsules, 0.32g / capsule, produced by Liaoning Kangchen Pharmaceutical Co., Ltd., batch number 150501. Retinoic acid tablets, 10mg / tablet, produced by Shandong Liangfu Pharmaceutical Co., Ltd., batch number 140230. The above medicines are prepared with distilled water to make a certain concentration for intragastric administration before use.
[0031] 1.3 Instruments and equipment
[0032] Atomic absorption spectrophotometer, single-photon bone mineral density tester, multifunctional true-color pathological image analyzer, electronic balance.
[0033] 2 Experimental methods
[0034] 2.1 Grouping and administration ...
Embodiment 2
[0053] Example 2 Pharmacodynamic study on prevention and treatment of steroid-induced necrosis of the femoral head by Wuling bacteria powder
[0054] 1. Materials
[0055] 1.1 Experimental animals
[0056] Wistar rat suckling mouse.
[0057] 1.2 Drugs and reagents
[0058] Wuling mushroom powder: provided by Hangzhou Zhongzhi Kanggu Biotechnology Co., Ltd., batch number JC16011401. Positive drug: Xianling Gubao capsule, 0.5g / capsule, Guizhou Tongjitang Pharmaceutical Co., Ltd., batch number 141203. Prednisone acetate tablets, 5mg / tablet, produced by Tianjin Pharmaceutical Group Xinzheng Co., Ltd., batch number 140516. The above medicines are prepared with distilled water to make a certain concentration for intragastric administration before use.
[0059] 2. Experimental method
[0060] Take Wistar rat suckling mice, half male and half male. It is randomly divided into 6 groups, 10 in every group, respectively blank control group, model group, positive control group (Xia...
Embodiment 3
[0070] Example 3 Pharmacodynamic study on prevention and treatment of alcoholic femoral head necrosis by Wuling bacteria powder
[0071] 1 material
[0072] 1.1 Experimental animals
[0073] 65 New Zealand male white rabbits aged 8-10 weeks in clean grade, body weight (2.5±0.5kg).
[0074] 1.2 Drugs and reagents
[0075] Wuling mushroom powder: provided by Hangzhou Zhongzhi Kanggu Biotechnology Co., Ltd., batch number JC16011401. Positive drug: Xianling Gubao capsule, 0.5g / capsule, Guizhou Tongjitang Pharmaceutical Co., Ltd., batch number 141203. The above medicines are prepared with distilled water to make a certain concentration for intragastric administration before use.
[0076] 2 Experimental methods
[0077] 8 mL / (kg·d) of strong liquor (containing 56% ethanol) was given by intragastric administration. After 4 weeks, 5 animals were randomly selected, and the pathological examination confirmed that the modeling was successful. The remaining 60 were randomly divided ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com