Preparation method of tin-based oxide with controllable components and photocatalytic application of tin-based oxide
A technology for oxides and water control, applied in the direction of metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problem of small adjustment space for product composition, high preparation cost, High equipment requirements, to achieve high-efficiency visible light photocatalytic degradation, decolorization activity, improved degradation rate, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
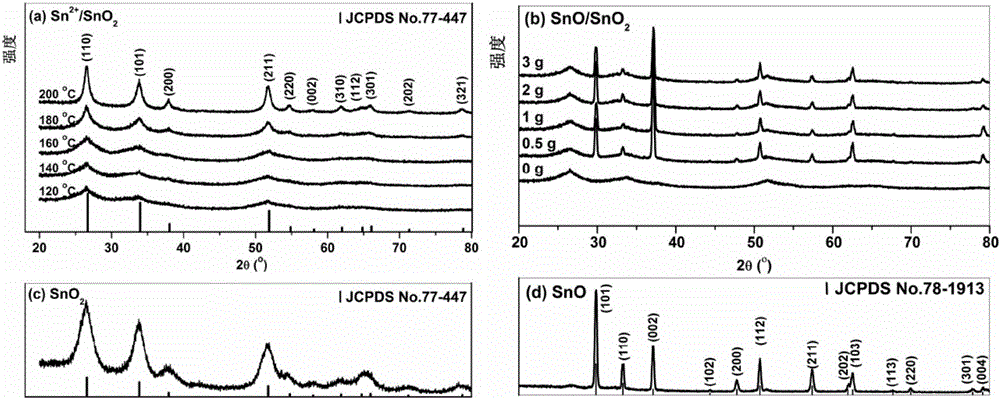
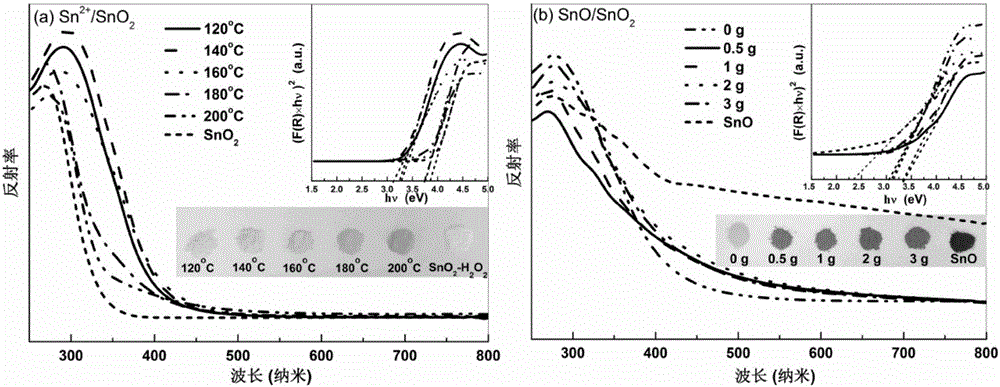
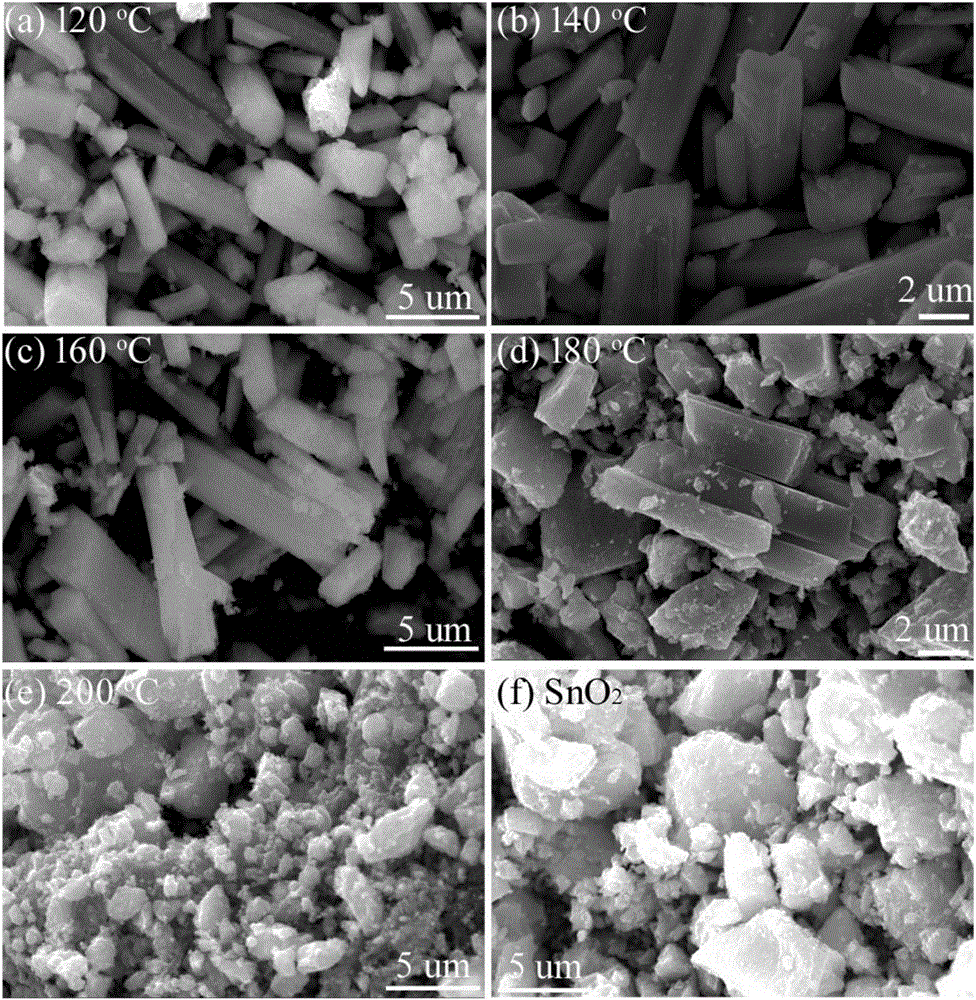
[0047] This embodiment prepares Sn according to the following steps 2+ / SnO 2 :
[0048] a. Take 1g SnCl 2 2H 2 O was added to a polytetrafluoroethylene container, then 80mL of water was added, and stirred until dissolved to obtain a hydrothermal solution; five samples were made in parallel;
[0049] b. Seal five polytetrafluoroethylene containers filled with hydrothermal solutions and put them into stainless steel hydrothermal kettles, and then place them in blast ovens at 120°C, 140°C, 160°C, 180°C, and 200°C respectively. After heat treatment for 24 hours, the product solution was obtained after natural cooling to room temperature;
[0050] c. The product solution is subjected to centrifugation, washing and vacuum drying at 80°C to obtain Sn 2+ / SnO 2 sample. The obtained sample was light yellow solid, and the color of the sample gradually deepened as the temperature increased.
Embodiment 2
[0052] This embodiment prepares SnO / SnO according to the following steps 2 :
[0053] a. Weigh 1g of SnCl with an electronic balance 2 2H 2 O was added to the polytetrafluoroethylene container, and 0.5g, 1g, 2g, 3g of urea were added respectively, then 80mL of water was added, stirred until dissolved, and a hydrothermal solution was obtained;
[0054] b. Seal the polytetrafluoroethylene container containing the hydrothermal solution and put it into a stainless steel hydrothermal kettle, then place it in a blast oven at 160°C for hydrothermal treatment for 24 hours, and naturally cool to room temperature to obtain the product solution;
[0055] c. The product solution is subjected to centrifugal separation, washing and vacuum drying at 80°C to obtain SnO / SnO 2 . The obtained sample is a gray-black solid, and the color of the sample gradually deepens as the amount of urea increases.
Embodiment 3
[0057] This embodiment prepares SnO according to the following steps 2 :
[0058] a. Take 1g SnCl 2 2H 2 0 and 3g of urea were added to the polytetrafluoroethylene container, then 80mL of water and 1mL of mass concentration were added to be 30% hydrogen peroxide, and the mixture was evenly stirred to obtain a hydrothermal solution;
[0059] b. Seal the polytetrafluoroethylene container containing the hydrothermal solution and put it into a stainless steel hydrothermal kettle, then place it in a blast oven at 160°C for hydrothermal treatment for 24 hours, and naturally cool to room temperature to obtain the product solution;
[0060] c. The product solution is subjected to centrifugal separation, washing and vacuum drying at 80°C to obtain SnO 2 . The obtained sample was a white powdery solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com